Five-membered heteroaromatics tolylene glucoside and preparation method and use thereof
A technology of glucopyranoside and heterocycle, which is applied in the field of type 2 sodium glucose transporter inhibitor and its preparation, and can solve problems such as liver toxicity and body weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 2-[(4-Amino-3-methylthio-1,3,4-triazol-5-yl)methyl]phenol (IIIa-1)
[0067]
[0068] Add 2.22g (10mmol) of compound IVa-1 and 20mL of methanol to a 50mL round bottom flask, add 0.40g (10mmol) of solid NaOH under stirring at room temperature, stir for 10 minutes, then add dropwise 1.26g (10mmol) of freshly distilled MeOH 2 SO 4 5 mL of methanol solution, after the dropwise addition, stirred at room temperature for 1 hour. The reaction mixture was poured into 200mL saturated brine, acidified with concentrated hydrochloric acid to pH=4-5, extracted with 50mL×3 chloroform, the organic phase was washed with saturated brine, anhydrous Na 2 SO 4 After drying, the solvent was evaporated on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IIIa-1. Colorless crystals, 1.91g, 81%. IR(KBr), 3421, 3328, 3245, 1597, 1503cm -1 .
[0069] Compounds IVa-1 and IIIa-1 are one of the compounds having the general formula IVa and ...
Embodiment 2
[0071] 3-[(4-Amino-3-ethylthio-1,3,4-triazol-5-yl)methyl]phenol (IIIa-2)
[0072]
[0073] Add 2.22g (10mmol) of compound IVa-2 and 20mL of methanol to a 50mL round bottom flask, add 0.40g (10mmol) of solid NaOH under stirring at room temperature, stir for 10 minutes, then add dropwise 1.54g (10mmol) of newly distilled Et 2 SO 4 5 mL of methanol solution, after the dropwise addition, stirred at room temperature for 1 hour. The reaction mixture was poured into 200mL saturated brine, acidified with concentrated hydrochloric acid to pH=4-5, extracted with 50mL×3 chloroform, the organic phase was washed with saturated brine, anhydrous Na 2 SO 4 After drying, the solvent was evaporated on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IIIa-2. Colorless crystals, 2.10g, 84%. IR(KBr), 3426, 3322, 3243, 1598, 1502cm -1 .
[0074] Compounds IVa-2 and IIIa-2 are one of the compounds having the general formula IVa and the g...
Embodiment 3
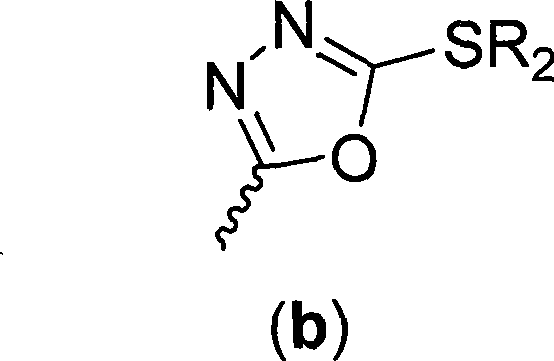
[0076] 2-[(2-Methylthio-1,3,4-oxadiazol-5-yl)methyl]phenol (IIIb-1)
[0077]
[0078] Add 2.08g (10mmol) of compound IVb-1 and 20mL of methanol to a 50mL round bottom flask, add 0.40g (10mmol) of solid NaOH under stirring at room temperature, stir for 10 minutes, then dropwise add 1.26g (10mmol) of freshly distilled MeOH 2 SO 4 5 mL of methanol solution, after the dropwise addition, stirred at room temperature for 1 hour. The reaction mixture was poured into 200mL saturated brine, acidified with concentrated hydrochloric acid to pH=4-5, extracted with 50mL×3 chloroform, the organic phase was washed with saturated brine, anhydrous Na 2 SO 4 After drying, the solvent was evaporated on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IIIb-1. Colorless crystals, 2.02g, 91%. IR(KBr), 3427, 1599, 1501cm -1 .
[0079] Compounds IVb-1 and IIIb-1 are one of the compounds having the general formula IVb and the general formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com