Implantable sensors and implantable pumps and anti-scarring agents
A sensor, scar technology, applied in the direction of organic active ingredients, peptide/protein ingredients, tetracycline active ingredients, etc., can solve problems such as inaccurate readings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
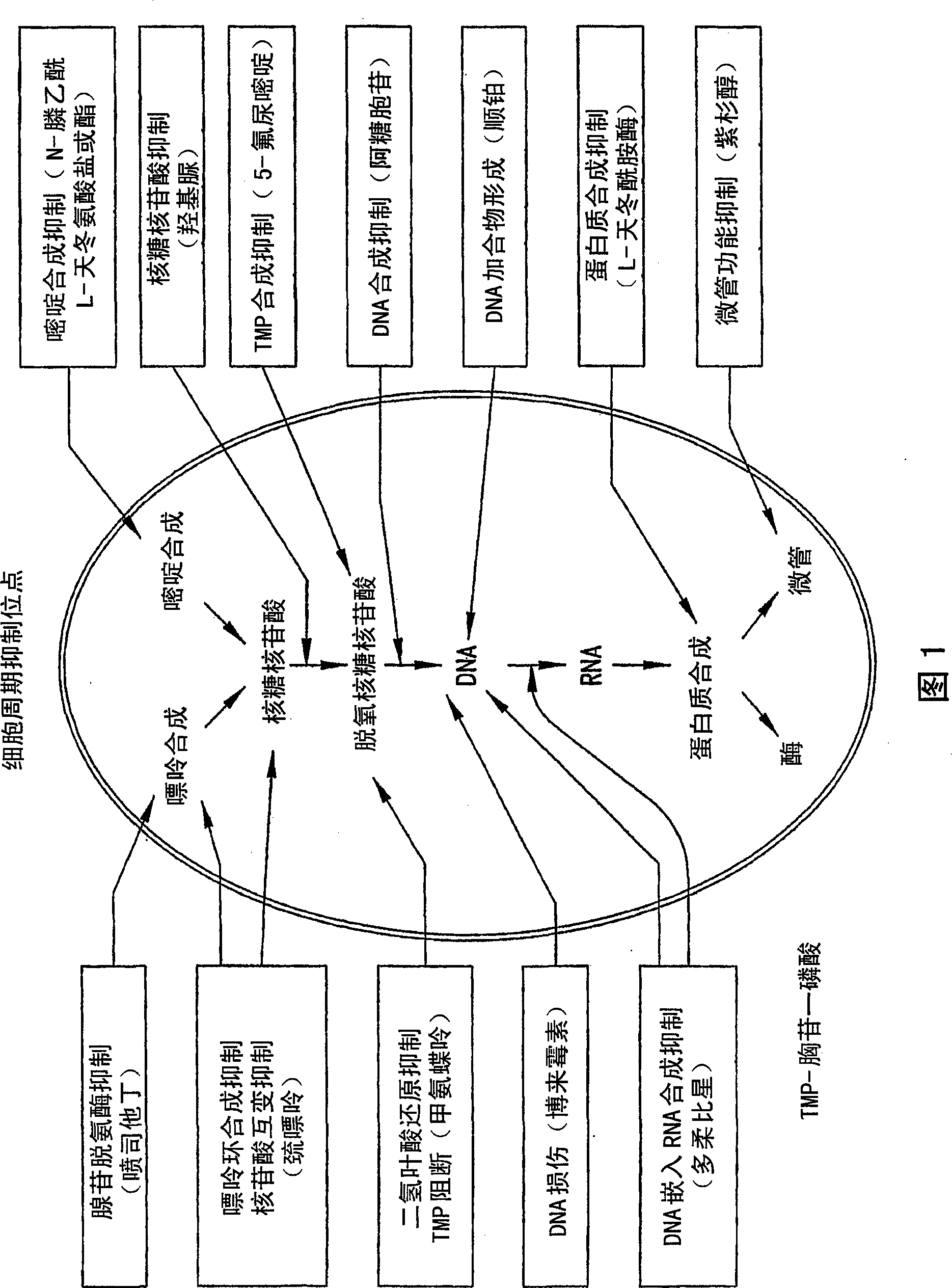
[0591] The present invention provides agents (eg, chemotherapeutic agents) that can be delivered from a composition and that have potent antimicrobial activity at very low doses. A variety of anti-infective agents can be used in combination with the compositions of the present invention. Suitable anti-infective agents can be readily determined based on the assay provided in Example 52. Discussed in more detail below are representative examples of some agents that may be used: (A) anthracyclines (e.g., doxorubicin and mitoxantrone), (B) fluoropyrimidines (e.g., 5-FU ), (C) folate antagonists (eg, methotrexate), (D) podophyllotoxins (eg, etoposide), (E) camptothecin, (F) hydroxyurea, and (G) platinum compounds (eg, cisplatin).
[0592] (A). Anthracyclines
[0593] Anthracyclines have the following general structures, where the R groups can be different organic groups:
[0594]
[0595] According to U.S. Patent No. 5,594,158, suitable R groups are as follows: R 1 for CH...
Embodiment 1
[1236] Washing is performed by immersing the metal part of the housing of the device (eg, MiniMed 2007 Implantable Insulin Pump, Medtronic, Inc.) in HPLC grade isopropanol. Use a parylene coater (for example, PDS 2010 LABCOATER 2 from Cookson Electronics) and di-p-xylylene (PARYLENE N) or dichloro-bis-p-xylylene ( PARYLENE D) (both available from Specialty Coating Systems, Indianapolis, IN) was used as the coating feed material to deposit a parylene primer layer (approximately 1 to 10 um) on clean equipment.
Embodiment 2
[1238] Paclitaxel Coating - Partial Coating
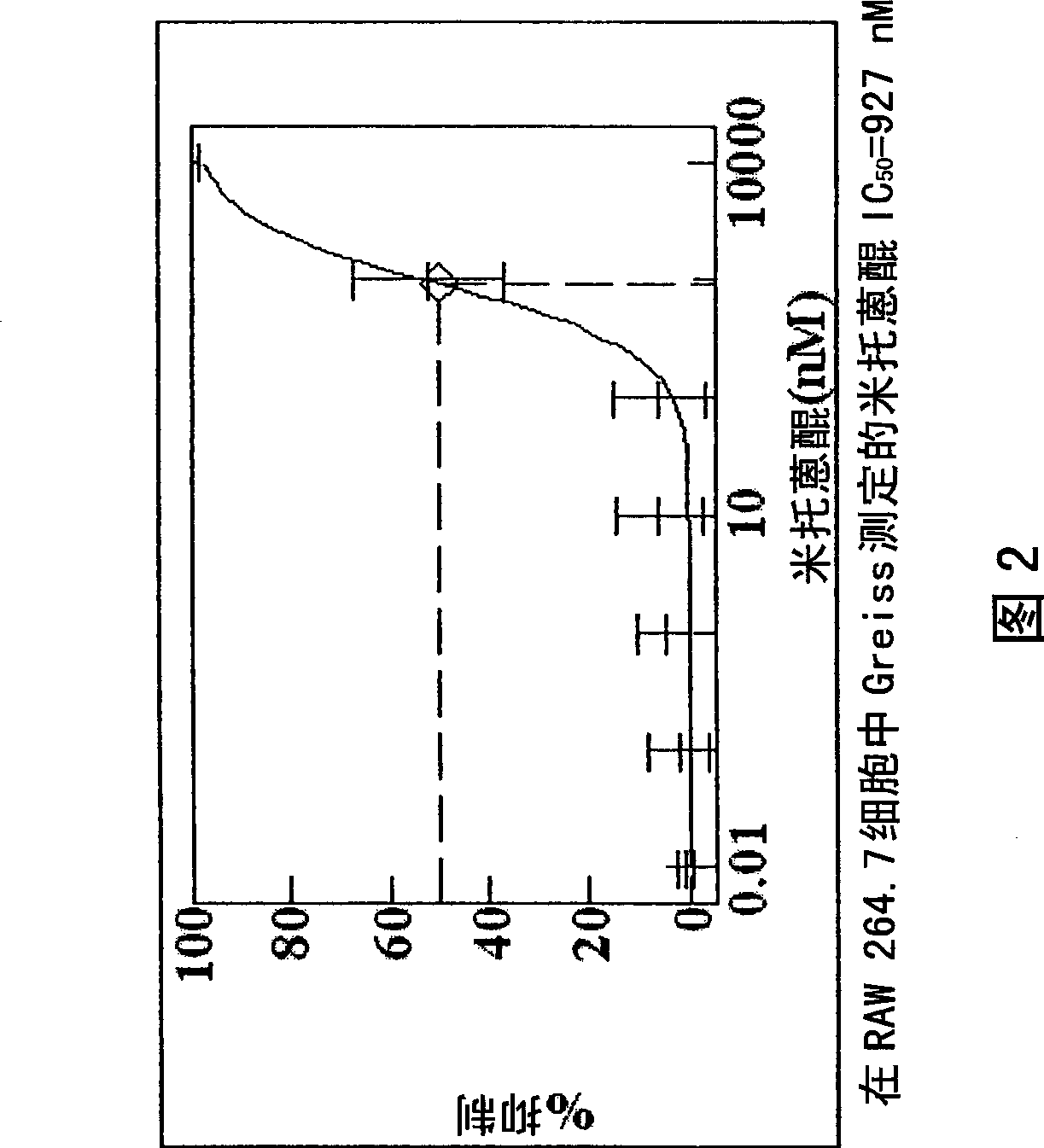
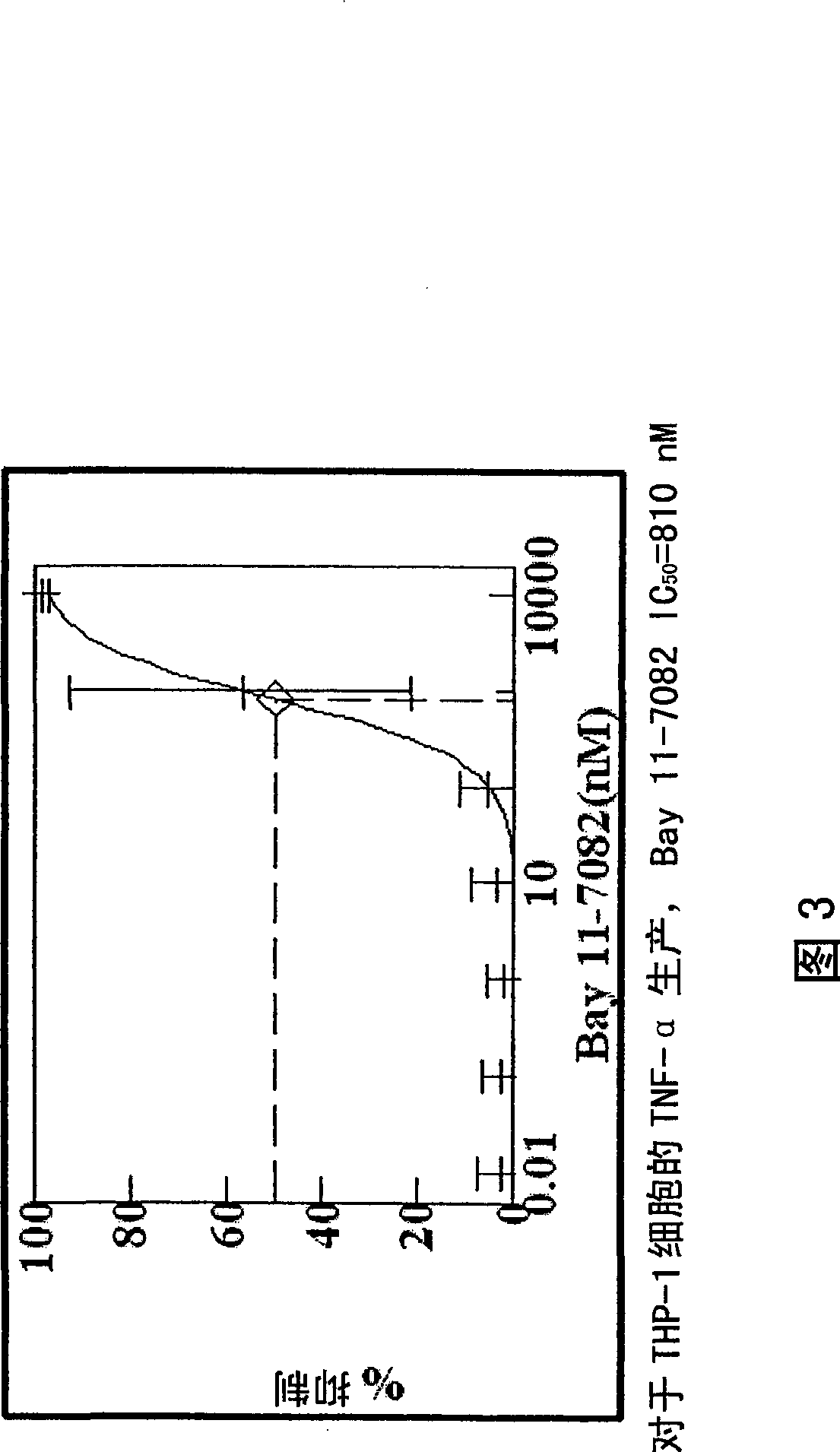
[1239] Paclitaxel solutions were prepared by dissolving paclitaxel (5 mg, 10 mg, 50 mg, 100 mg, 200 mg and 500 mg) in 5 ml HPLC grade THF. The coated portion of the parylene-coated device (as prepared, eg, in Example 1) was immersed in the paclitaxel / THF solution. After the selected incubation time, the devices were removed from the solution and dried in a forced air oven (50°C). The device was then dried overnight in a vacuum oven. The amount of paclitaxel and the incubation time used for each solution were varied so that the amount of paclitaxel coated on the device was 0.06 μg / mm 2 to 10μg / mm 2 (μg Paclitaxel / mm 2 device, which was placed in THF / paclitaxel solution and then coated with paclitaxel). The time the device is kept in the paclitaxel / THF solution can be varied, with longer soak times generally providing more adsorption of paclitaxel to the device. In another example, paclitaxel was replaced with one of the follow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com