Paeoniflorin crystallization process with controllable crystal form and granularity
A technology of azithromycin and particle size, which is applied in the crystallization process field of azithromycin, can solve the problems of inability to meet the polymorphic requirements of azithromycin, large fluctuations in particle size distribution, optimization of preparation methods, etc., to achieve stable preparation of crystal forms and good gloss , the effect of regular crystal appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
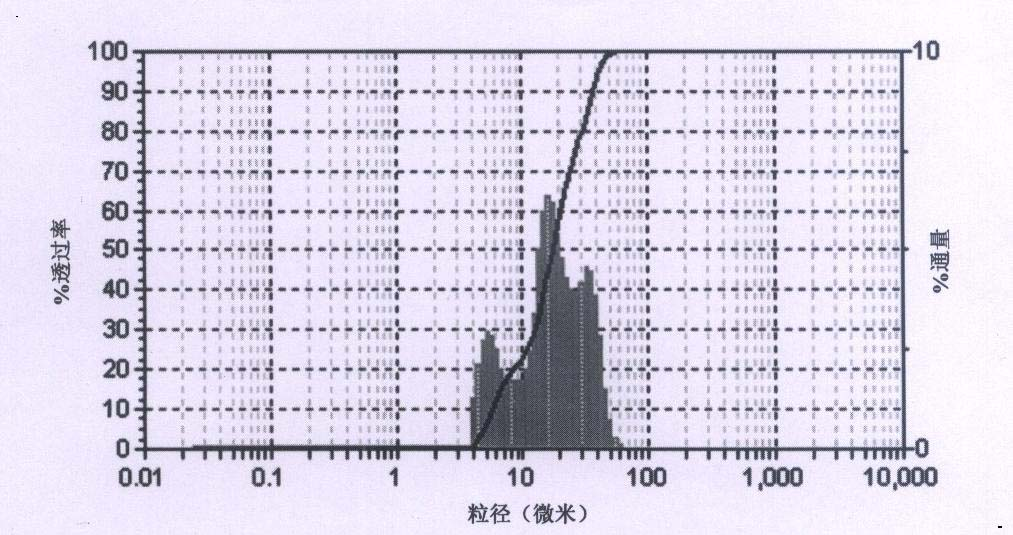
[0035] Dissolve 20g of the amorphous azithromycin raw material in 170mL of absolute ethanol, adjust the pH value to 10.0, set the volume to 200mL, put it into a 1000mL three-neck flask, keep the constant temperature water bath at 40°C, and control the stirring speed at 500r·min -1 , add 2% sodium chloride of azithromycin quality. Then start to add pure water slowly, and the flow rate is controlled to be 20% azithromycin organic solution per hour. The initial volume is slightly turbid. After stopping the flow and adding crystal growth for 2 hours, continue to maintain the same flow rate and continue to add until 500mL is added. Pure water, suction filtration, vacuum, in the presence of desiccant anhydrous calcium chloride, and dried at 40°C for 4 hours. The finally obtained azithromycin crystal form has good stability (unit cell parameters are shown in Table 3), regular appearance, crystal form purity of 99%, main particle size of 40.3 μm, distribution width of 21.5 μm, and wat...
Embodiment 2
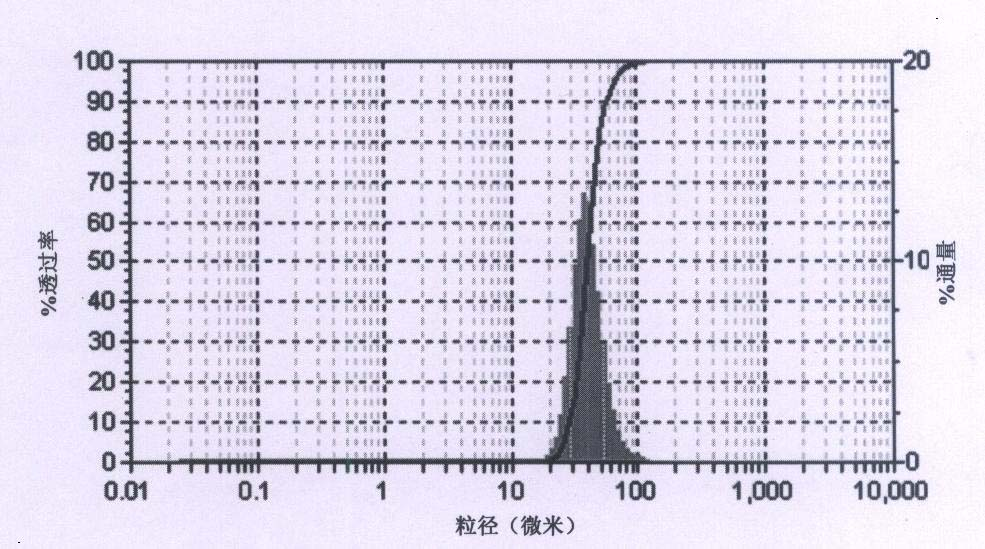
[0037] Dissolve 24g of amorphous azithromycin raw material in 170mL of ethylene glycol, adjust the pH value to 11.0, set the volume to 200mL and put it into a 1000mL three-neck flask, keep the constant temperature water bath at 20°C, and control the stirring speed at 300r·min -1 , add 2% sodium acetate by mass of azithromycin. Then start to add pure water slowly, and the flow rate is controlled at an initial volume of 15% azithromycin organic solution per hour, which is slightly turbid. After stopping the flow and adding crystal growth for 2 hours, continue to maintain the same flow rate and continue to feed until the flow is completed 500mL Purified water, filtered by suction, dried under vacuum at 40°C for 5 hours in the presence of desiccant silica gel. The final crystal form of azithromycin (see Table 3 for unit cell parameters) has a regular appearance, a crystal form purity of 99%, a main particle size of 72 μm, a distribution width of 44.2 μm, and a water content of 3.2...
Embodiment 3
[0039] Dissolve 30g of the amorphous azithromycin raw material in 170mL of tetrahydrofuran, adjust the pH value to 8.0, set the volume to 200mL, put it into a 1000mL three-neck flask, keep the constant temperature water bath at 30°C, and control the stirring speed at 500r·min -1 , add 2% sodium chloride by mass of azithromycin. Then start to add pure water slowly, and the flow rate is controlled at an initial volume of 15% azithromycin organic solution per hour, which is slightly turbid. After stopping the flow and adding crystal growth for 2 hours, continue to maintain the same flow rate and continue to feed until the flow is completed 500mL Pure water, suction filtration, vacuum, in the presence of desiccant anhydrous magnesium nitrate, and dried at 40°C for 8 hours. The final crystal form of azithromycin (see Table 3 for unit cell parameters) has a regular appearance, a crystal form purity of 99%, a main particle size of 32.4 μm, a distribution width of 20.2 μm, and a water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com