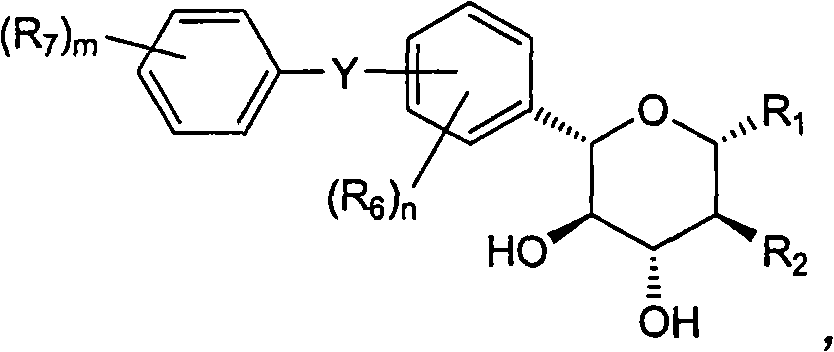
Inhibitors of sodium glucose co-transporter 2 and methods of their use
A technology of SO2 and SO2R1A, which can be used in medical preparations containing active ingredients, sugar derivatives, organic chemistry, etc., and can solve problems such as chemical space difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1: (2S, 3R, 4R, 5S)-2-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-6-methoxy-tetrahydro-pyran- Synthesis of 3,4,5-triol
[0153]
[0154] The title compound was prepared by the following steps.
[0155] a. [(3aS, 5S, 6R, 6aS)-6-(tert-butyl-dimethyl-silyloxy)-2,2-dimethyl- Tetrahydro-furo[2,3-d][1,3]dioxol-5-yl]-methanol Preparation: This compound was synthesized using methods known in the art. See for example, Nucleosides Nucleotides , 20:649-652 (2001) and references therein.
[0156] b. (3aS, 5R, 6R, 6aS)-6-(tert-butyl-dimethyl-silyloxy)-2,2-dimethyl- Tetrahydro-furo[2,3-d][1,3]dioxole-5-carbaldehyde Preparation: In N2 CH of oxalyl chloride (0.76ml, 8.7mmol) at -78°C 2 Cl 2 (55ml) dropwise added DMSO (0.84ml, 11.8mmol) in CH 2 Cl 2 (5ml) solution. After 15 minutes, CH containing the alcohol from step A (2.40 g, 7.9 mmol) was added dropwise. 2 Cl 2 (20ml). After 15 minutes, slowly add NEt 3 . The reaction was slowly warmed to room temperature o...
Embodiment 2
[0162] Example 2: (3S, 4R, 5R, 6S)-6-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-tetrahydro-pyran-2,3,4, Synthesis of 5-tetrol
[0163]
[0164] The alcohol (51 mg, 0.093 mmol) from step D of Example 1 was dissolved in a sealed vial at 80 °C with 1:1 AcOH:H 2 O (1 ml) was treated for 18 hours. The reaction was cooled to room temperature, diluted with EtOAc to transfer to a flask, and concentrated in vacuo. The residue was dissolved in CH 2 Cl 2 , with NaHCO 3 and MgSO 4 Worked for 30 minutes, filtered and concentrated in vacuo. The product was purified by flash chromatography (4 g SiO 2 , 0-12%MeOH:CH 2 Cl 2 , 30 minutes, 10ml / min), suspended in H 2 O and lyophilized to give (3S,4R,5R,6S)-6-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-tetrahydro-pyran-2, 3,4,5-tetraol (31 mg, 0.079 mmol, 85%), as a white solid. NMR showed a 1:1 ratio of α and β anomers.
[0165] 1 H NMR (400MHz, methanol-d 4 )δppm 7.34 (dd, J=8.08, 4.04Hz, 1H), 7.22-7.30 (m, 2H), 7.09 (d, J=8.34Hz, 2H), 6...
Embodiment 3
[0166] Example 3: (2S, 3R, 4R, 5S)-2-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-6-ethoxy-tetrahydro-pyran- Synthesis of 3,4,5-triol
[0167]
[0168] A 0.35 M HCl solution in EtOH was prepared by adding AcCl (0.025 ml, 0.35 mmol) to EtOH (1 ml) and stirring for 15 minutes. The alcohol from Example 1, Step D (61 mg, 0.11 mmol) was treated with this solution in a sealed vial for 2 hours at 80°C. The reaction was cooled to room temperature and washed with concentrated NH 4 OH quenched until basic, with NaHCO 3 Treat for 30 min with CH 2 Cl 2 Diluted, filtered and concentrated in vacuo. The product was purified by flash chromatography (4 g SiO 2 , 0-10%MeOH:CH 2 Cl 2 , 40 minutes, 10ml / min), suspended in H 2 O and lyophilized to give (2S,3R,4R,5S)-2-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-6-ethoxy-tetrahydro -pyran-3,4,5-triol (40 mg, 0.095 mmol, 85%) as a white solid. NMR showed a 1.75:1 ratio of α and β anomers.
[0169] 1H NMR (400MHz, chloroform-d) δppm: 7.28-7.32 (m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com