Salt of sulfinylbenzimidazole compound, and crystal and amorphous form thereof
A technology of sulfinyl and benzimidazole, which is applied in the field of salts of sulfinyl benzimidazole compounds, their crystals and amorphous substances, and can solve undisclosed problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
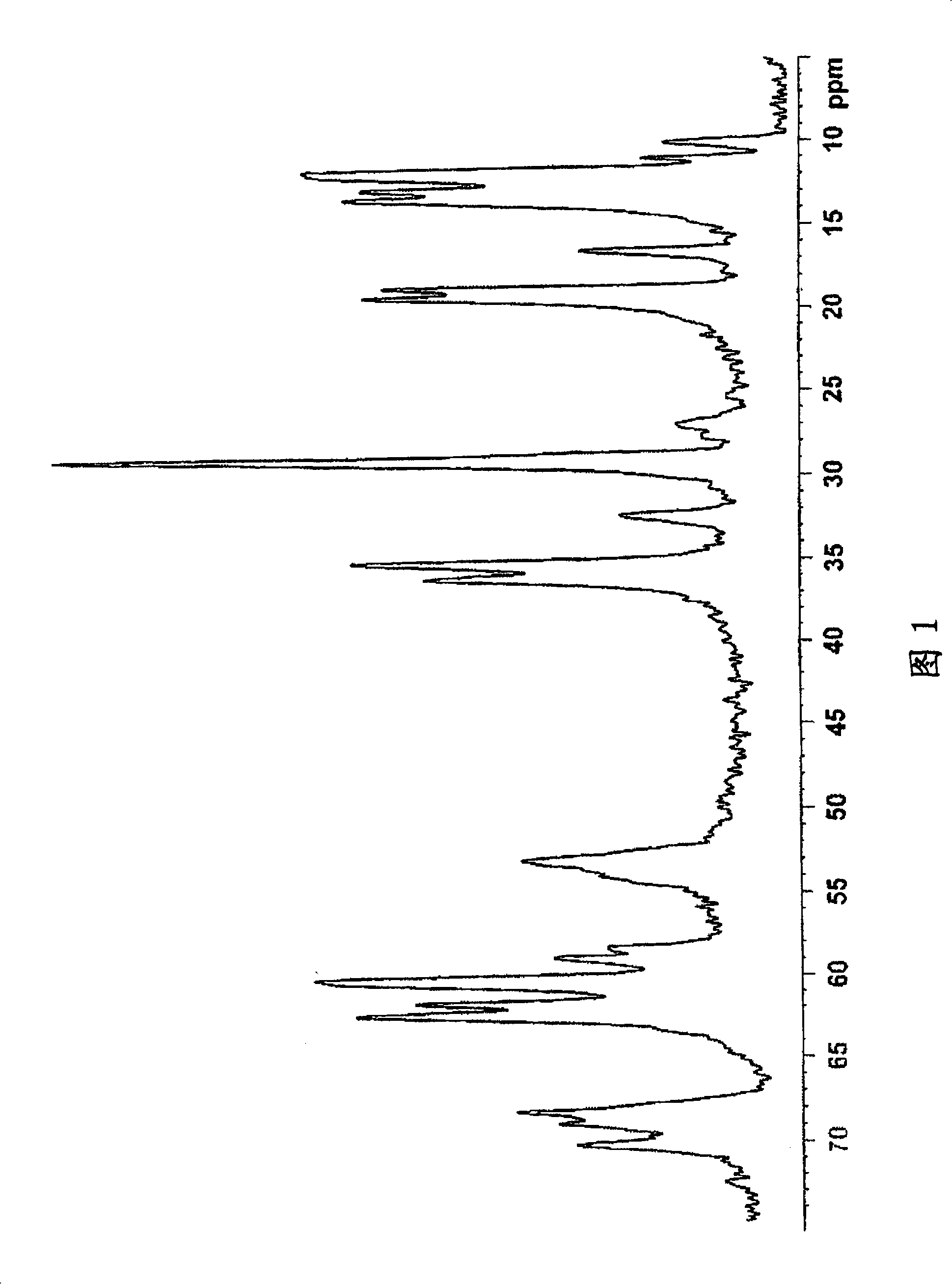
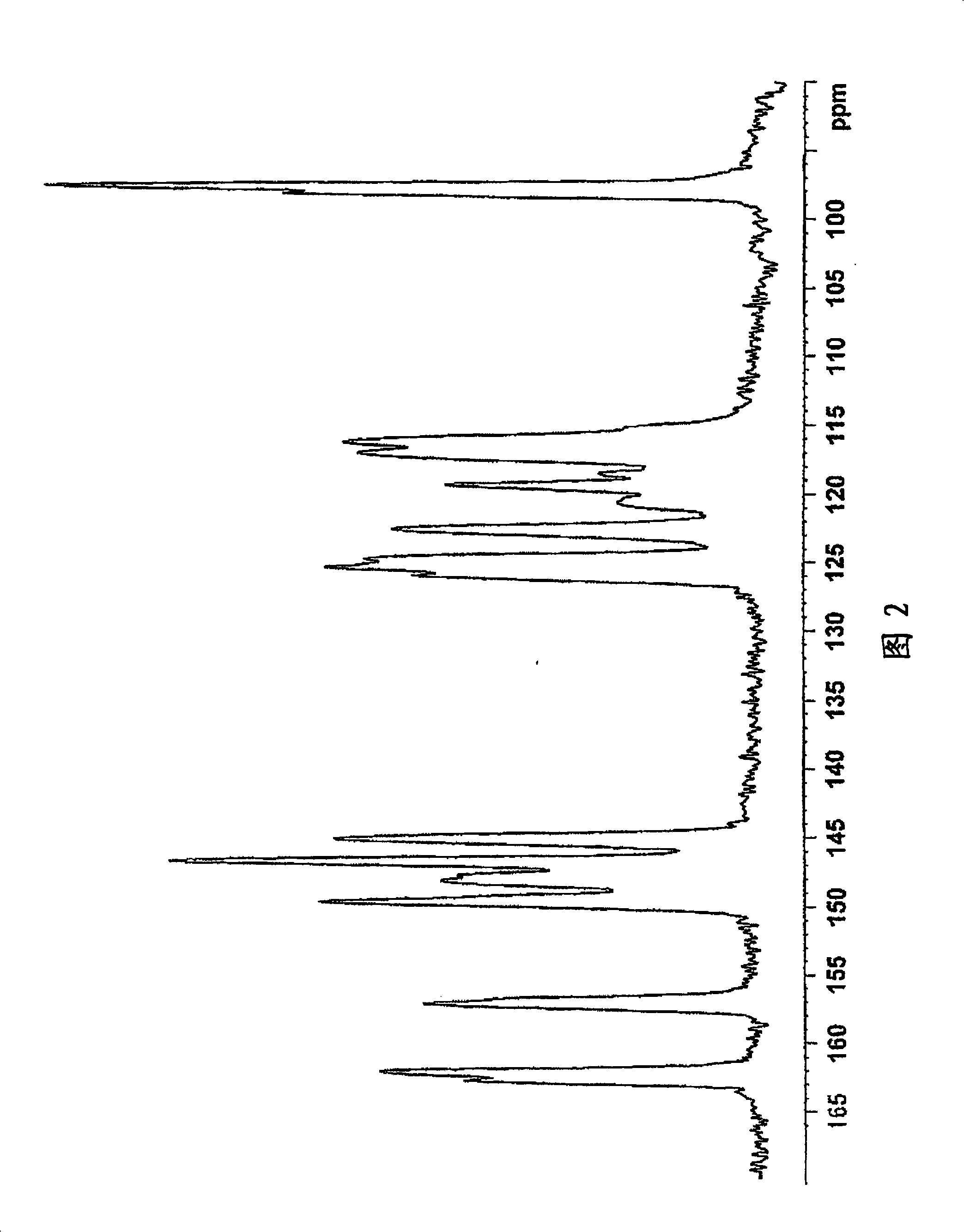
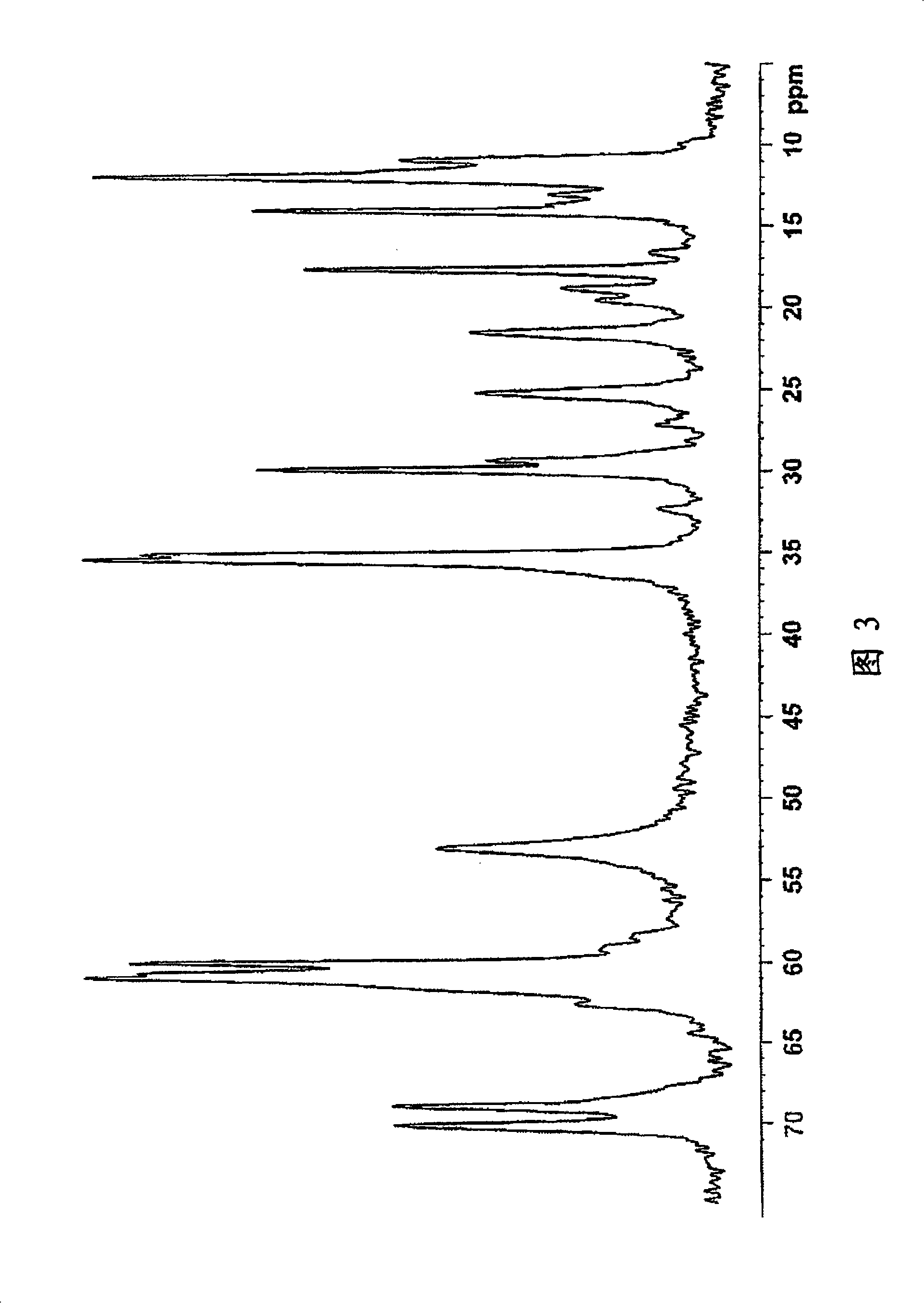
Image
Examples
manufacture example 1
[0298] (Production Example 1) (2,2-Dimethyl-1,3-dioxan-5-yl)methanol
[0299]
[0300] A mixture of 2-(hydroxymethyl)-1,3-propanediol (4.09g, 38.5mmol), acetone (130ml, 1768mmol), and 70% perchloric acid (1.37g, 9.55mmol) was stirred at room temperature for 21 hours . After the reaction mixture was adjusted to pH=9 with concentrated aqueous ammonia, it was concentrated. The residue was purified by silica gel column chromatography (silica gel: 100 g, elution solvent: heptane, heptane / ethyl acetate = 1 / 3) to obtain the title compound (4.83 g, yield: 85.8%) as a colorless oil. .
[0301] 1 H NMR (400MHz, DMSO-d 6 )δppm; 1.29 (3H, s), 1.30 (3H, s), 1.64-1.74 (1H, m), 3.35-3.41 (2H, m), 3.61 (2H, dd, J=7, 12Hz), 3.82 ( 2H,dd,J=4,12Hz), 4.54(1H,t,J=5Hz).
manufacture example 2
[0302] (Production Example 2) 2,3,5-collidine 1-oxide
[0303]
[0304] To acetic acid (1.43 kg, 23.83 mol) was added 2,3,5-collidine (1.43 kg, 11.80 mol) over 15 minutes. After 15 minutes, 35% hydrogen peroxide water (1.38 kg, 14.2 mol) was added dropwise over 30 minutes, followed by stirring overnight at 90°C to 95°C. Sodium sulfite (220 g) was added to the reaction liquid. The reaction mixture was poured into a mixture of sodium carbonate (2.5 kg) and water (12 L), and extracted with chloroform (3.0 L×4). The resulting organic layer was concentrated until crystals precipitated, and n-hexane (2.5 L) was added to the precipitate, followed by stirring overnight under ice-cooling. The obtained crystals were filtered to obtain 1.53 kg of the target substance.
manufacture example 3
[0305] (Production Example 3) 2,3,5-Trimethyl-4-nitropyridine 1-oxide
[0306]
[0307] To 98% sulfuric acid (4.93 kg, 49.3 mol) was added 2,3,5-collidine 1-oxide (1.38 kg, 10.1 mol). After dripping 97% nitric acid (1.44 kg) over 50 minutes, it heated at 85 degreeC for 4 hours. The reaction solution was added to a mixture of ammonium bicarbonate (10.6 kg) and water (9.0 L), and extracted with ethyl acetate (3.0 L×3). The resulting organic layer was concentrated and vacuum-dried overnight to obtain 1.50 kg of the desired substance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| light receiving slit | aaaaa | aaaaa |
| light receiving slit | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com