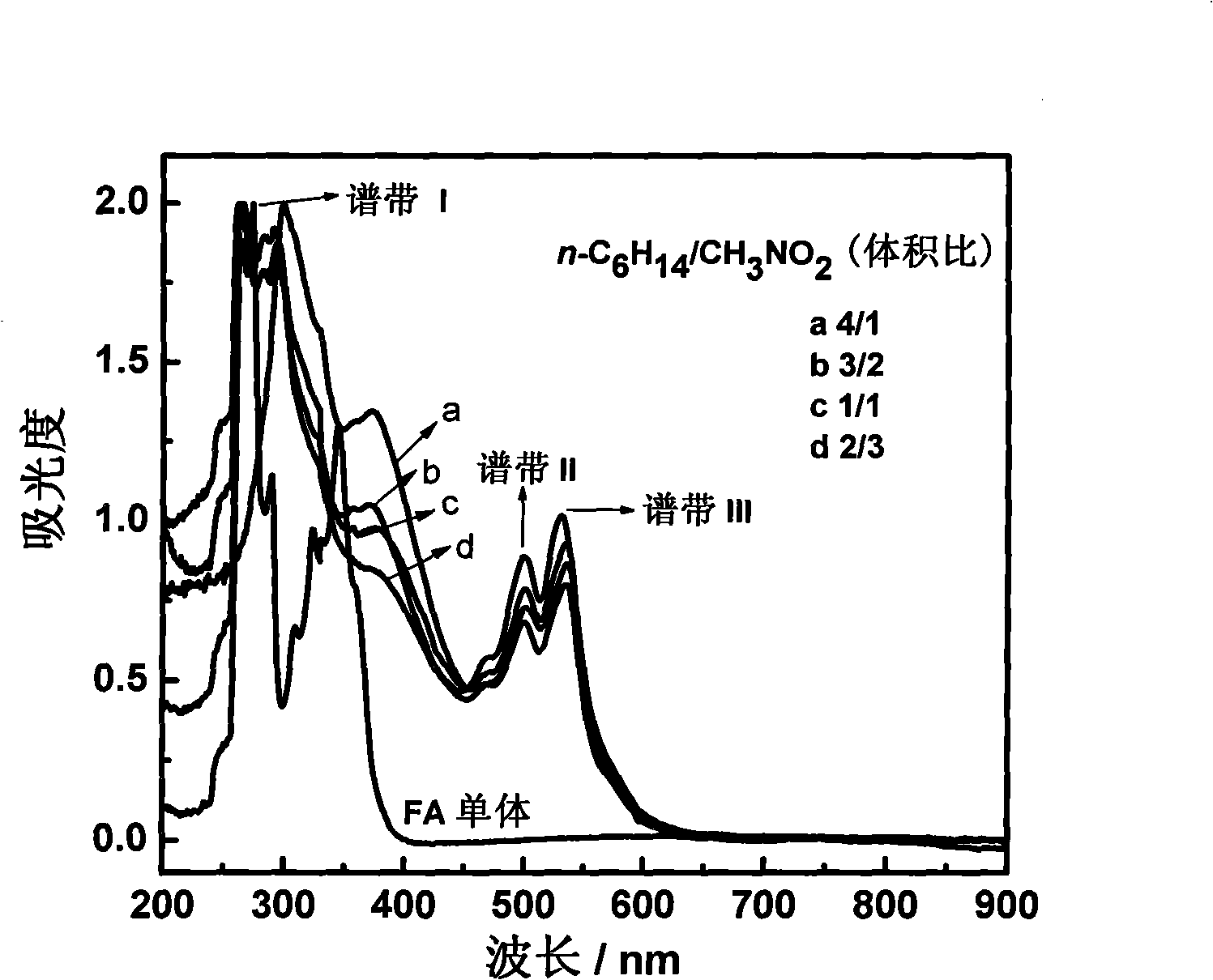
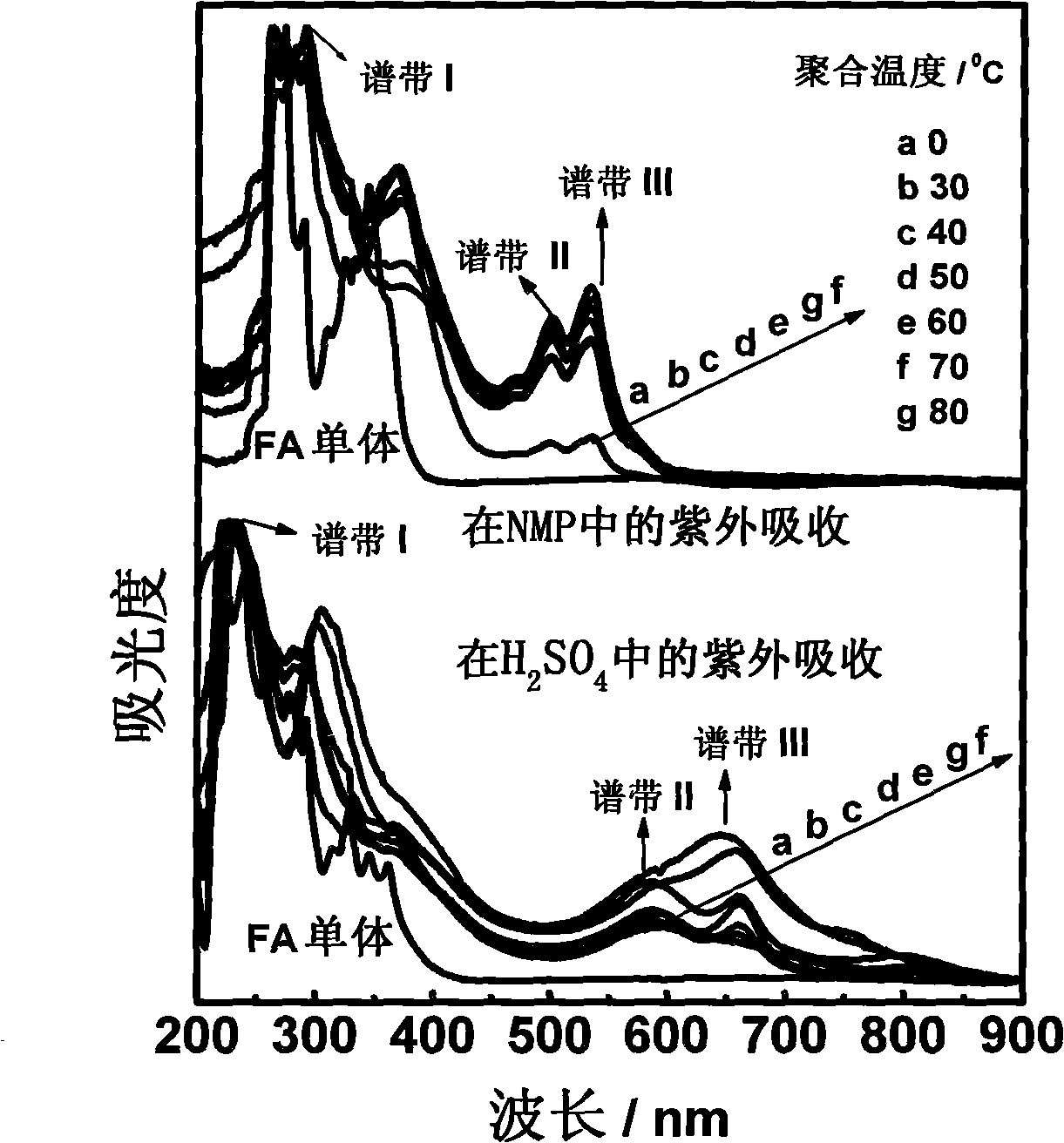
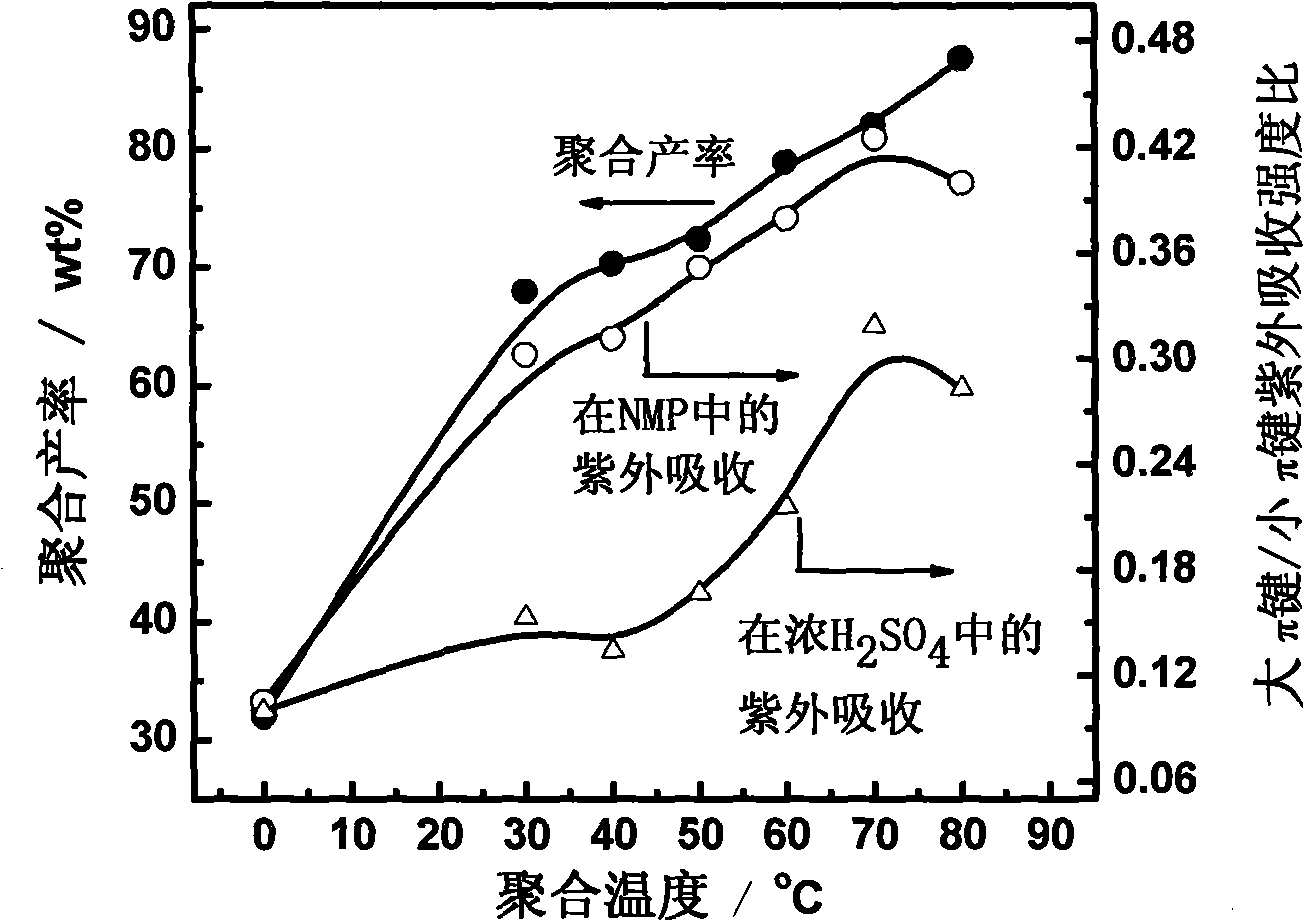
Method for preparing polyfluoranthene in two-phase system
A dual-phase system, polyfluoranthene technology, which is applied in the field of preparing polyfluoranthene, can solve the problems of reducing polymer functionality and its application potential, etc., and achieves the effects of simple and easy operation, easy removal, and improved yield and performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Add 417 mg (2 mmol) of FA monomer into 15 mL of n-hexane solvent, and then ultrasonically disperse it for 5-10 min to promote the complete dissolution of FA monomer to prepare a monomer solution. Dissolve 2270.8mg (14mmol) of anhydrous ferric trichloride in 10mL of nitromethane solvent, and then ultrasonically disperse it for 10-15min to promote the complete dissolution of ferric oxide to prepare an oxidizing agent solution. After the two solutions were kept at 70° C. for 30 minutes, the monomer solution was added dropwise to the above-mentioned oxidant solution which was continuously stirred. The reaction was continued for 24 h in a 70 °C water bath. The liquid surface of the solution was stratified, changing from reddish brown to black, and black precipitate was formed. After the reaction, the solution was suction-filtered, and then the product was washed with absolute ethanol several times to remove residual monomers and oligomers, and then the obtained product was ...
Embodiment 2
[0060] Add 417 mg (2 mmol) of FA monomer into 12.5 mL of n-hexane solvent, and then ultrasonically disperse it for 5-10 min to promote the complete dissolution of FA monomer to prepare a monomer solution. Dissolve 2270.8mg (14mmol) of anhydrous ferric trichloride in 12.5mL of nitromethane solvent, and then disperse it ultrasonically for 10-15min to promote the complete dissolution of ferric oxide to prepare an oxidizing agent solution. After the two solutions were kept at 70° C. for 30 minutes, the monomer solution was added dropwise to the above-mentioned oxidant solution which was continuously stirred. The reaction was continued for 24 h in a 70 °C water bath. The liquid surface of the solution was stratified, changing from reddish brown to black, and black precipitate was formed. After the reaction, the solution was suction-filtered, and then the product was washed with absolute ethanol several times to remove residual monomers and oligomers, and then the obtained product ...
Embodiment 3
[0062] Add 417 mg (2 mmol) of FA monomer into 10 mL of n-hexane solvent, and then ultrasonically disperse it for 5-10 minutes to promote the complete dissolution of FA monomer to prepare a monomer solution. Dissolve 2270.8mg (14mmol) of anhydrous ferric trichloride in 15mL of nitromethane solvent, and then disperse it ultrasonically for 10-15min to promote the complete dissolution of ferric oxide to prepare an oxidizing agent solution. After the two solutions were kept at 70° C. for 30 minutes, the monomer solution was added dropwise to the above-mentioned oxidant solution which was continuously stirred. The reaction was continued for 24 h in a 70 °C water bath. The liquid surface of the solution was stratified, changing from reddish brown to black, and black precipitate was formed. After the reaction, the solution was suction-filtered, and then the product was washed with absolute ethanol several times to remove residual monomers and oligomers, and then the obtained product ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com