Cocaine-and amphetamine-regulated transcript peptides single-chain antibody and application thereof
A technology for regulating transcription and single-chain antibodies, applied in the direction of antibodies, peptides, specific peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
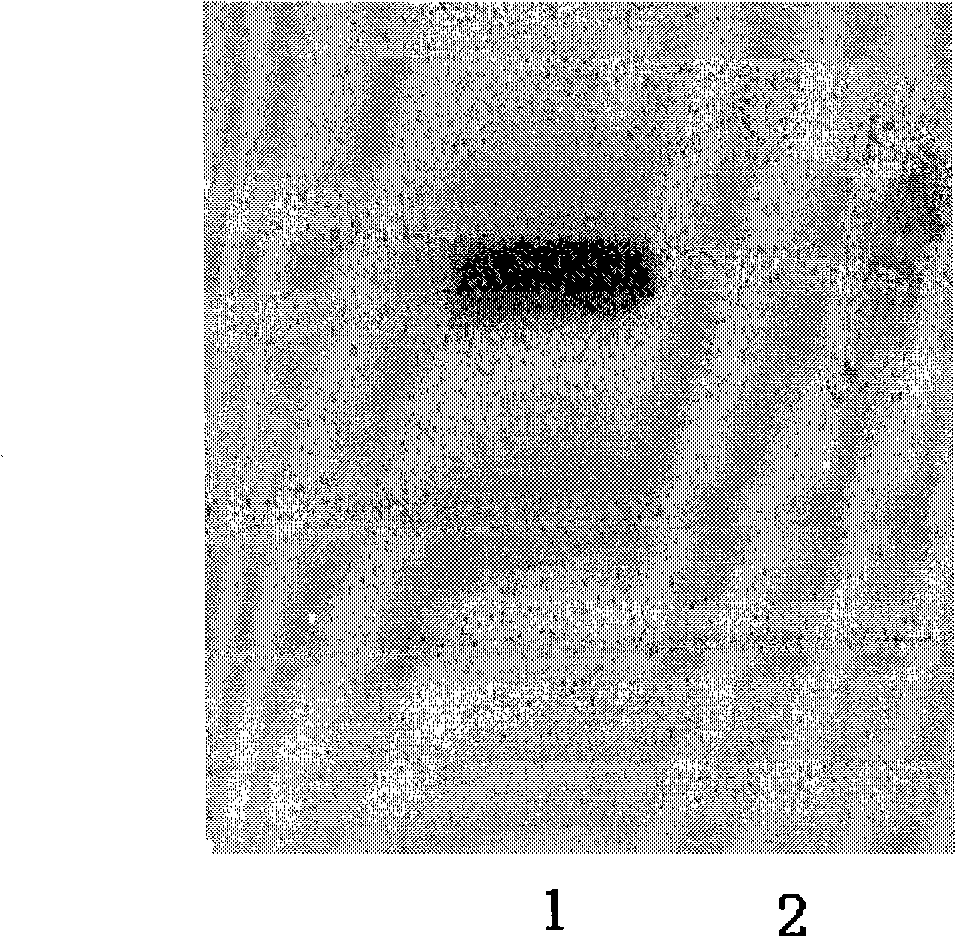
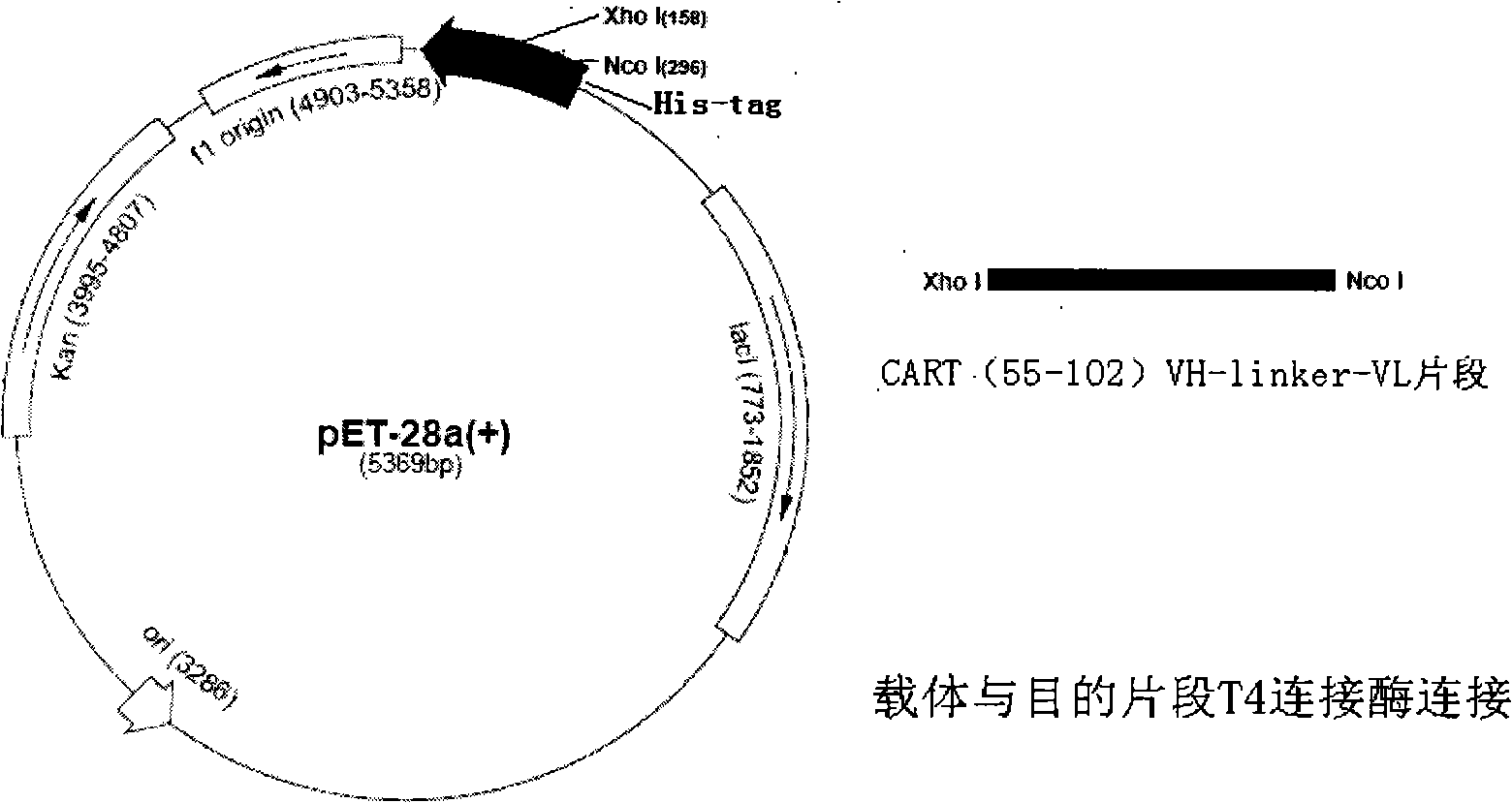
[0033] pGEX-4T3-CART 55-102 (Lin Qin, Cocaine-amphetamine-regulated transcription peptide antibody inhibits the formation and reconstruction of morphine-induced mouse conditioned place preference, 2006, Master's thesis of Second Military Medical University, pGEX-4T3 was purchased from Amersham phaemacia company) expressing glycerol bacteria 30μl transfer In 3ml of LB medium containing 100μg / ml, cultivate overnight at 37°C on a shaker; add 1ml of the transfer bacteria solution to 100ml of LB medium containing 100μg / ml Amp, grow on a shaker at 37°C until the OD600 value is 0.4, add 1M IPTG 400μl to make the final concentration 0.4mM, 37°C, 230r.p.m. shaker to induce expression for 5h, 4°C low-temperature centrifuge at 12,000r.p.m. for 20min to collect the bacteria, add PBS to suspend the bacteria at 10ml / wet weight (g) . Suspend the bacteria overnight in an ice bath, sonicate at 100W for 1 second, rest for 1 second, and sonicate for 20 minutes. Centrifuge at 12,000r.p.m. at 4℃...
Embodiment 2
[0035] Example 2: Effect of CART single-chain antibody (AH36) on cocaine-induced drug dependence
[0036] The antibody was used in animal experiments, and it was confirmed that the cocaine-amphetamine regulatory transcription peptide antibody reduces the drug dependence of the body caused by the use of cocaine and other psychotropic drugs. The result is as follows:
[0037] (1) Behavioral Sensitization Model
[0038] 1. Spontaneous Activity Recording Devices
[0039] The conditional place preference shuttle box black box used in this experiment was used as the spontaneous activity recording device. The black box is placed in a 100×100×100 soundproof box with an inner diameter of 15x15x35cm, and a piece of smooth barbed wire is placed 5cm above the bottom of the box; the shuttle door is closed. The observation box has the effect of light insulation and sound insulation, and the sound intensity attenuation is 20-25dB. On the top of the box, white lighting and infrared LEDs a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com