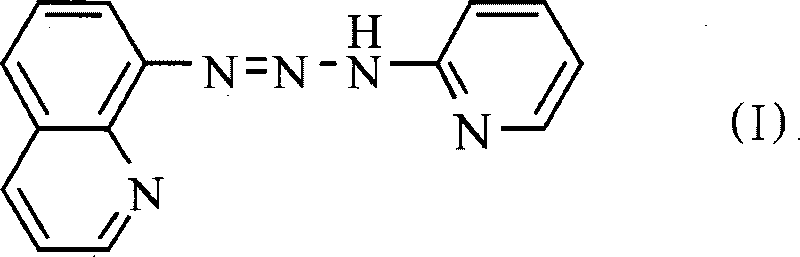
1-(8-chinoline)-3(2-pyridine)-triazene, preparation thereof and application thereof
A technology of triazene and quinoline, which is applied in the direction of chemical reaction of materials for analysis, fluorescence/phosphorescence, chemiluminescence/bioluminescence, etc., can solve the problems of triazene reagent interference, etc., and achieve high sensitivity and preparation Simple method and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
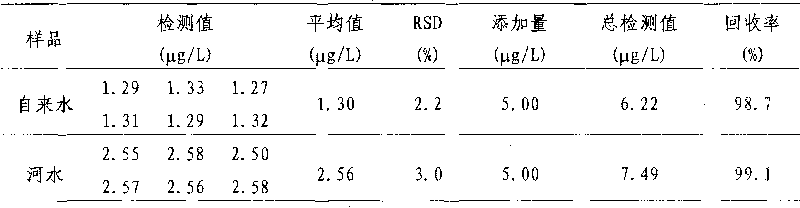
Image
Examples
Embodiment 1
[0020] 1) Diazotization of 8-aminoquinoline
[0021] Dissolve 0.36g (0.0025mol) 8-aminoquinoline in 0.5mL formic acid and 2.5mL concentrated sulfuric acid. Under stirring, slowly add 2.5mL 0.069g / mL sodium nitrite aqueous solution at 0℃, and react for 30min to diazo Completely.
[0022] 2) Preparation of 1-(8-quinoline)-3-(2-pyridine)-triazene
[0023] 0.25 g (0.00266 mol) of 2-aminopyridine was dissolved in 20 mL of ethanol, and the diazonium salt solution was slowly added with stirring at 0°C, during which the pH of the mixed solution was adjusted to 6 with saturated sodium carbonate solution, and the reaction was carried out for 2 hours. Let stand overnight, filter with suction, and wash with water and ethanol successively. After drying, it was recrystallized twice with 95% ethanol to obtain pure 1-(8-quinoline)-3-(2-pyridine)triazene (QPyT) with a yield of 65%.
[0024] The product has been tested by elemental analysis, infrared spectroscopy and nuclear magnetic resonance spectr...
Embodiment 2
[0026] 1) Diazotization of 8-aminoquinoline
[0027] Dissolve 0.576g (0.004mol) 8-aminoquinoline in 2.0mL acetic acid and 2.5mL concentrated sulfuric acid. Under stirring, slowly add 3.0mL 0.069g / mL sodium nitrite aqueous solution at 2°C, and react for 40min to diazo Completely.
[0028] 2) Preparation of 1-(8-quinoline)-3-(2-pyridine)-triazene
[0029] 0.235 g (0.0025 mol) of 2-aminopyridine was dissolved in 20 mL of ethanol, and the diazonium salt solution was slowly added with stirring at 3° C., during which the pH value of the mixed solution was adjusted to 6.5 with saturated sodium acetate solution, and the reaction was carried out for 2.5 hours. Let stand overnight, filter with suction, and wash with water and ethanol successively. After drying, it was recrystallized twice with 95% ethanol to obtain pure 1-(8-quinoline)-3-(2-pyridine)triazene (QPyT) with a yield of 60%.
[0030] After elemental analysis, infrared spectroscopy and nuclear magnetic resonance spectroscopy, it is ...
Embodiment 3
[0032] 1) Diazotization of 8-aminoquinoline
[0033] Dissolve 0.432g (0.004mol) 8-aminoquinoline in 1.5mL formic acid and 5.0mL concentrated sulfuric acid. Under stirring, slowly add 3.0mL 0.069g / mL sodium nitrite aqueous solution at 0℃, and react for 50min to diazo Completely.
[0034] 2) Preparation of 1-(8-quinoline)-3-(2-pyridine)-triazene
[0035] 0.376 g (0.003 mol) of 2-aminopyridine was dissolved in 30 mL of ethanol, and the above diazonium salt solution was slowly added with stirring at 4°C, during which the pH of the mixed solution was adjusted to 7 with saturated sodium acetate solution, and the reaction was carried out for 2 hours. Let stand overnight, filter with suction, and wash with water and ethanol successively. After drying, it was recrystallized twice with 95% ethanol to obtain pure 1-(8-quinoline)-3-(2-pyridine)triazene (QPyT) with a yield of 50%.
[0036] The element analysis, infrared spectroscopy and nuclear magnetic resonance spectroscopy are consistent with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com