Method for preparing raw medicament of stable fenpyroximate
A technology of pyraclofen and tert-butyl, which is applied in the field of special-effect products for mites, and can solve problems such as the sales of pyraclofen active drug by Japan Agricultural Chemicals Co., Ltd.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
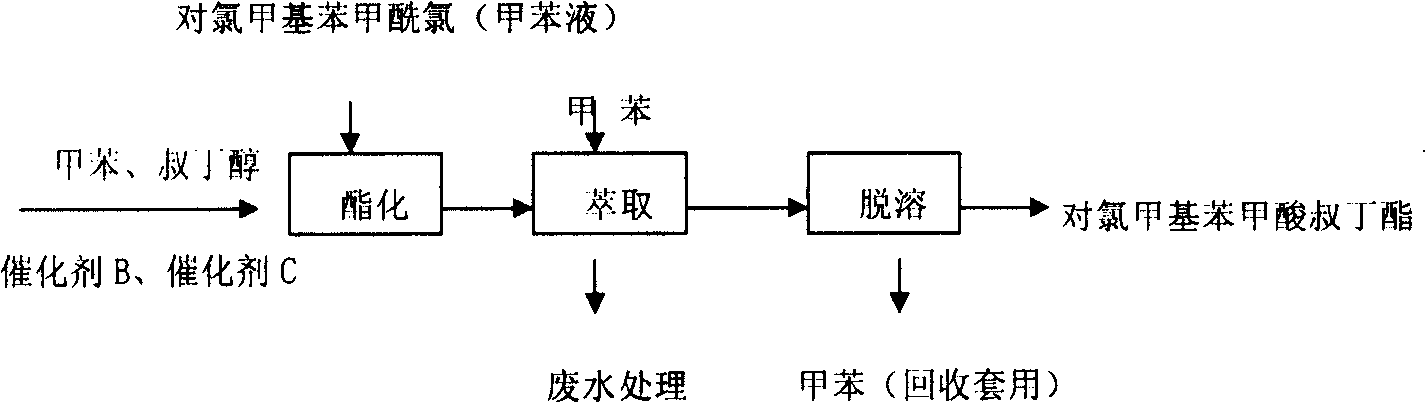
[0014] The synthesis of pyraclofen is mainly: the synthesis of two intermediates of tert-butyl p-chloromethylbenzoate and 1,3-dimethyl-5-phenoxypyrazole-4-carboxylic acid aldoxime, and then the two The intermediate synthesis is a finished product of pyraclofen with a purity of ≥96%.
[0015] (1) Synthesis of tert-butyl p-chloromethylbenzoate
[0016] The synthesis of tert-butyl p-chloromethylbenzoate is mainly divided into three stages, which are respectively the synthesis of p-chloromethylbenzoic acid, p-chloromethylbenzoyl chloride, and tert-butyl p-chloromethylbenzoate.
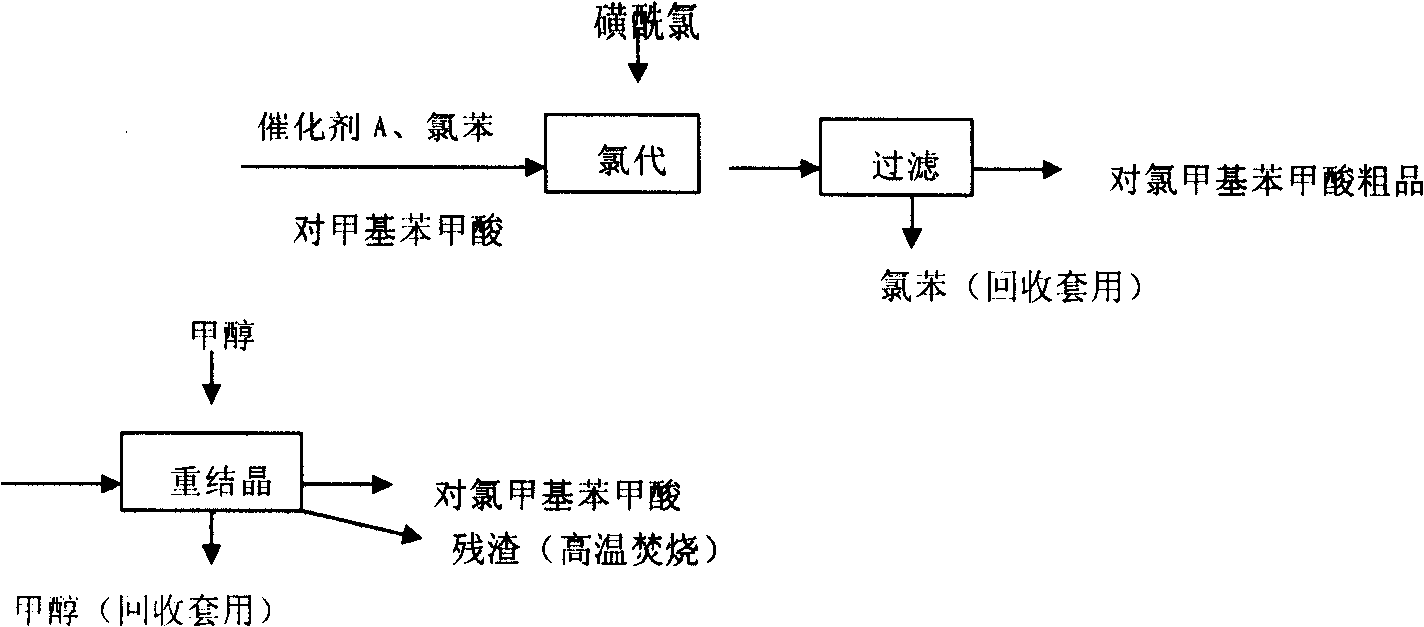
[0017] 1) Synthesis of p-chloromethylbenzoic acid
[0018] Add chlorobenzene, catalyst A, and p-toluic acid into the reaction kettle, add sulfuryl chloride dropwise under reflux, drop the temperature after the dropwise addition, keep warm, filter, and recrystallize methanol to obtain p-chloromethylbenzoic acid.
[0019] ① Reactive formula
[0020]
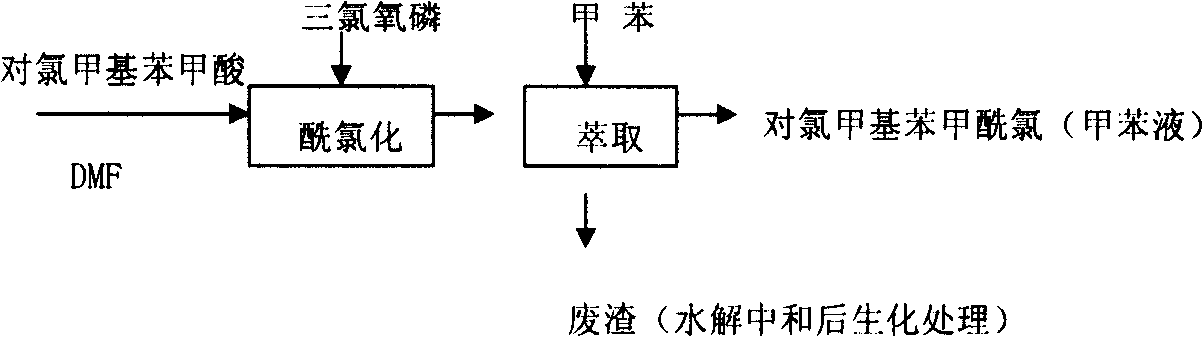
[0021] 2) Synthesis of p-chloromethylbenzoyl chlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com