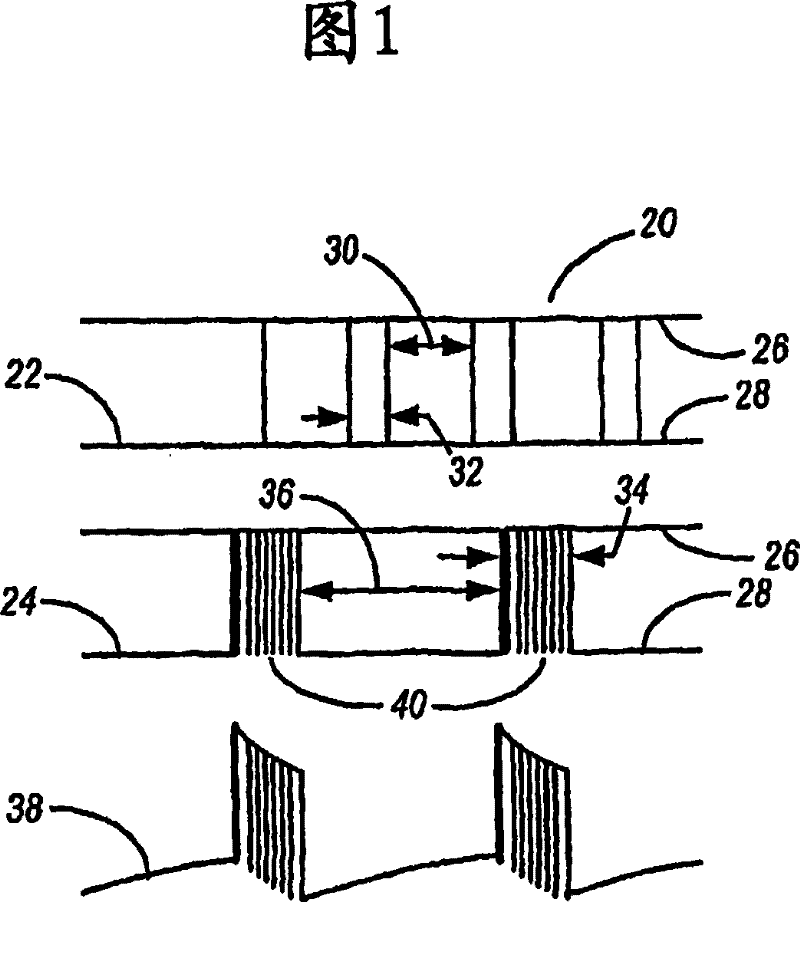
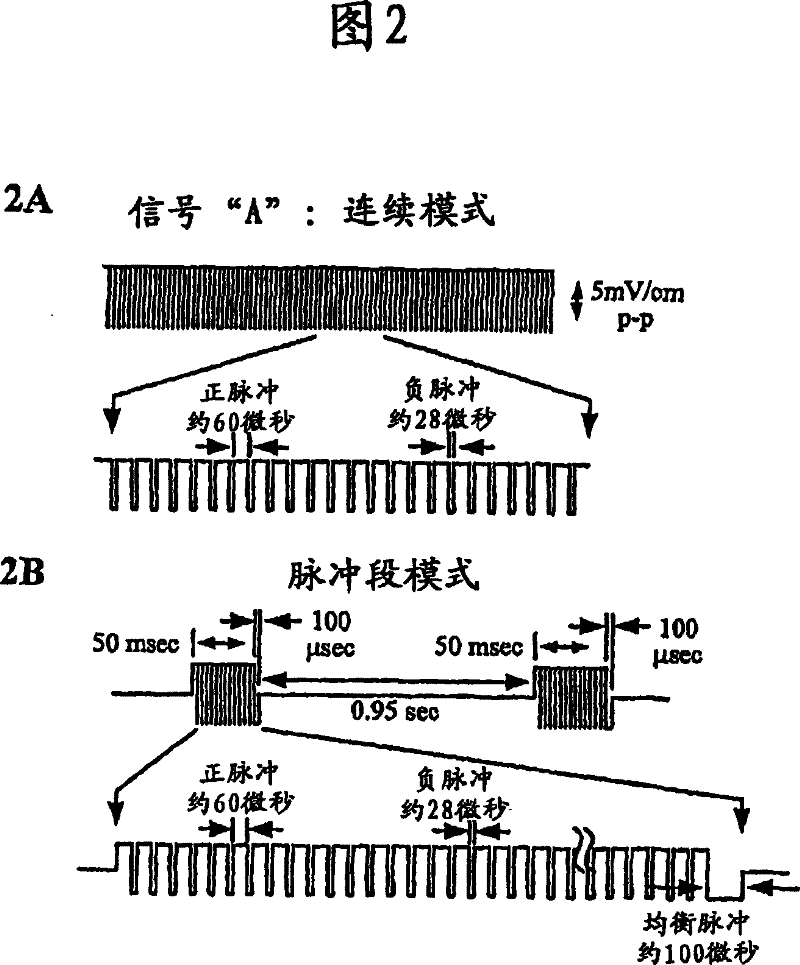
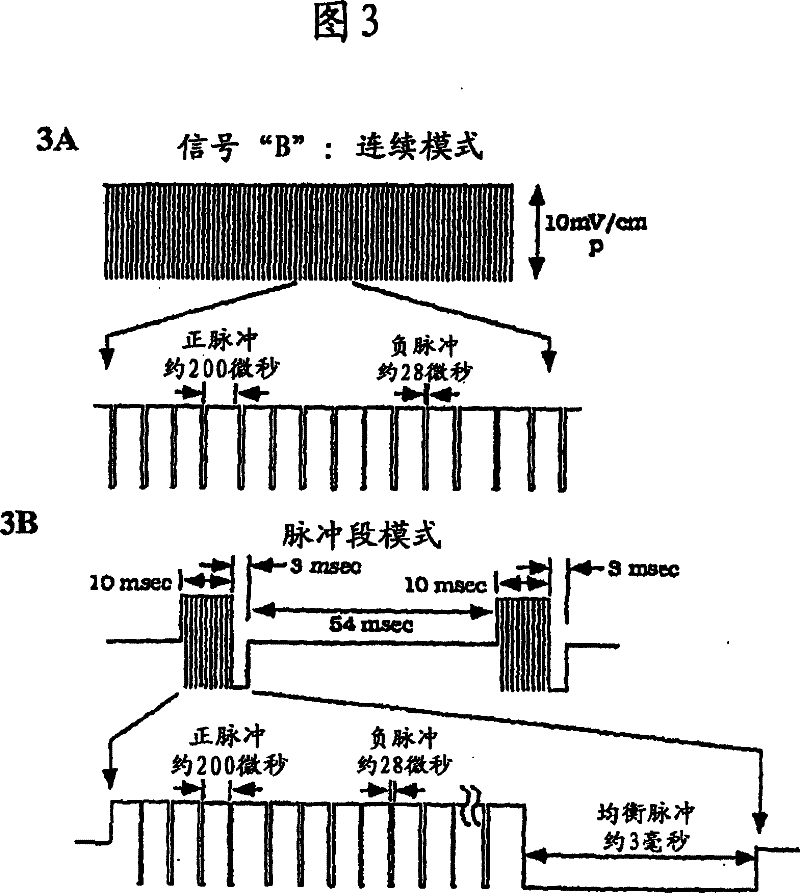
Methods for modulating chondrocyte proliferation using pulsing electromagnetic fields
A pulse and cartilage technology, applied in electrotherapy, treatment, etc., can solve the problems of large circuit complexity, input power, high price, and inability to provide signals, and achieve the effect of promoting growth and repair, low cost, and lasting healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Effects of PEMF signaling structure on mineralization and morphology in primary osteoblast cultures
[0142] The goal of this study was to compare two PEMF waveform structures delivered by capacitive coupling by evaluating biochemical and morphological changes in primary osteocyte cultures.
[0143] method
[0144] Osteoblast culture: primary human osteoblasts (CAMBREX , Walkersville, MD) was amplified to 75% confluence (confluence), and seeded directly on the previously described LAB-TEK at a density of 50,000 cells / ml TM (NALGENUNC INTERNATIONAL TM, Rochester, NY) room. Cultures were initially supported on basal osteoblast medium in the absence of differentiation factors. When the cultures reached 70% confluency in house, the medium was supplemented with hydrocortisone-21-hemisuccinate (200 mM final concentration), β-glycerophosphate (10 mM final concentration) and ascorbic acid. At 37°C, 5% CO 2 , Osteoblasts were cultured in humid air under 95% air for 21 day...
Embodiment 2
[0160] Use of niobium "salt" bridges for PEMF stimulation in vitro
[0161] introduction
[0162] Development of passive electrode systems using anodized niobium wires to couple time-varying electrical signals into the culture chamber. The purpose of this design is to replace the traditional electrolyte used to transmit PEMF-like signals (such as those generated capacitively rather than inductively by the EBI repetitive pulse segment bone growth stimulator in in vitro tissue for cell and tissue research). bridge technology to reduce complexity and improve reproducibility. Anodized niobium wire is readily available and requires only simple hand tools to form the electrode bridge. At the available frequencies (typically between 5Hz and 3Hz), the passage of DC current is negligible.
[0163] background
[0164] Capacitively coupled electric fields have generally been introduced into culture media with traditional electrolyte salt bridges, which have limited frequency response...
Embodiment 3
[0174] Stimulation of chondrocytes using capacitively coupled PEMF signals
[0175] introduction
[0176] Clinical applications of PEMF signaling similar to those used for bone repair are currently being tested for their ability to reduce joint pain in arthritic patients. Of interest is whether this pain-reducing signal can also ameliorate the underlying problem of damaged cartilage.
[0177] background
[0178] Compared to drug treatments and biologics, PEMF-based therapeutics offer an easy-to-use non-invasive treatment that contains no foreign agents with potential side effects and has zero washout time. Problems with PEMF therapeutic approaches include: identifying responding cells, elucidating the physical transduction site on the cell, and determining the biological mechanism of the response leading to the cellular response. The aim of this study was to identify the specific PEMF signal currently being tested for pain reduction (MEDRELIEFE , Healthonics, Inc, GA) can...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com