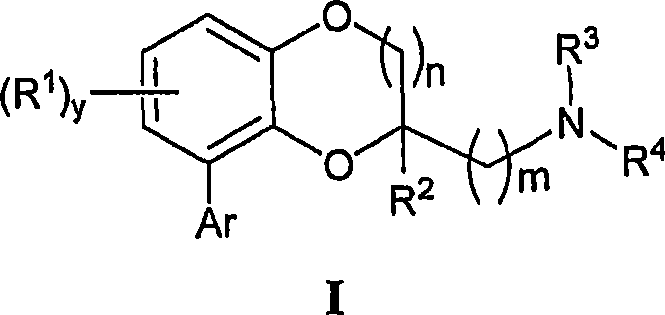
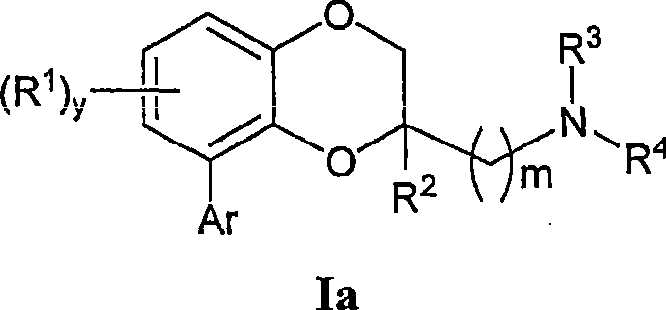
Benzodioxane and benzodioxolane derivatives and uses thereof
A phenyl, monocyclic technology, applied in the field of 5-HT2C receptor agonists or partial agonists, can solve the problem of not reducing the inflammation of the substantia nigra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0799] {[8-(2-chlorophenyl)-2,3-dihydro-1,4-benzodi-en-2-yl]methyl}amine: 2-azidomethyl-8-( 2-Chloro-phenyl)-2,3-dihydro-benzo[1,4]di-ene (100mg, 0.33mmol) and 5% Pt-S 2 Carbon (0.25 g) in methanol (50 mL) was hydrogenated overnight in a Parr apparatus at 55-60 Psi. The mixture was filtered through a pad of celite. The solvent was removed in vacuo to form a colorless oil. Dissolving the oil in ethanol converted to white solid fumarate (37mg); mp 210-211°C;
[0800] MS(ES)m / z 276[M+H] +
[0801] to C 15 h 14 ClNO 2 ·C 4 h 4 o 4 Elemental analysis of:
[0802] Theoretical values: C, 58.25; H 4.63; N, 3.57
[0803] Found values: C, 57.81; H, 4.58; N, 5.67
[0804] General procedure for the production of I from azide derivatives:
[0805] To a solution of the intermediate azide (1.0 mmol) in tetrahydrofuran was added polymer-supported triphenylphosphine (-3 mmol / g, 2.0 mmol) and water. The mixture was stirred at room temperature for 24 hours and filtered through a p...
Embodiment 2
[0808]{[8-(2-fluorophenyl)-2,3-dihydro-1,4-benzodi-en-2-yl]methyl}amine: with 2-azidomethyl-8-( Starting from 2-fluoro-phenyl)-2,3-dihydro-benzo[1,4]di-ene (140 mg, 0.5 mmol), 87 mg (47%) of the title compound were obtained as fumarate; mp188 -190°C;
[0809] MS(ESI)m / z 260[M+H] +
[0810] to C 15 h 14 FNO 2 ·C 4 h 4 o 4 Elemental analysis of:
[0811] Theoretical value: C, 60.80; H, 4.83; N, 3.73
[0812] Found values: C, 61.14; H, 4.42; N, 3.74
Embodiment 3
[0814] {[8-(2-methylphenyl)-2,3-dihydro-1,4-benzodi-en-2-yl]methyl}amine: with 2-azidomethyl-8- Starting from (2-methyl-phenyl)-2,3-dihydro-benzo[1,4]di-ene (110 mg, 0.39 mmol), 42 mg (29%) of the title compound were obtained as fumaric acid Salt, mp 201-202°C; MS (ESI) m / z 256 [M+H] + .
[0815] to C 16 h 17 NO 2 ·C 4 h 4 o 4 Elemental analysis of:
[0816] Theoretical values: C 64.68; H 5.70; N 3.77
[0817] Found: C, 64.70; H 5.46; N 3.71
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com