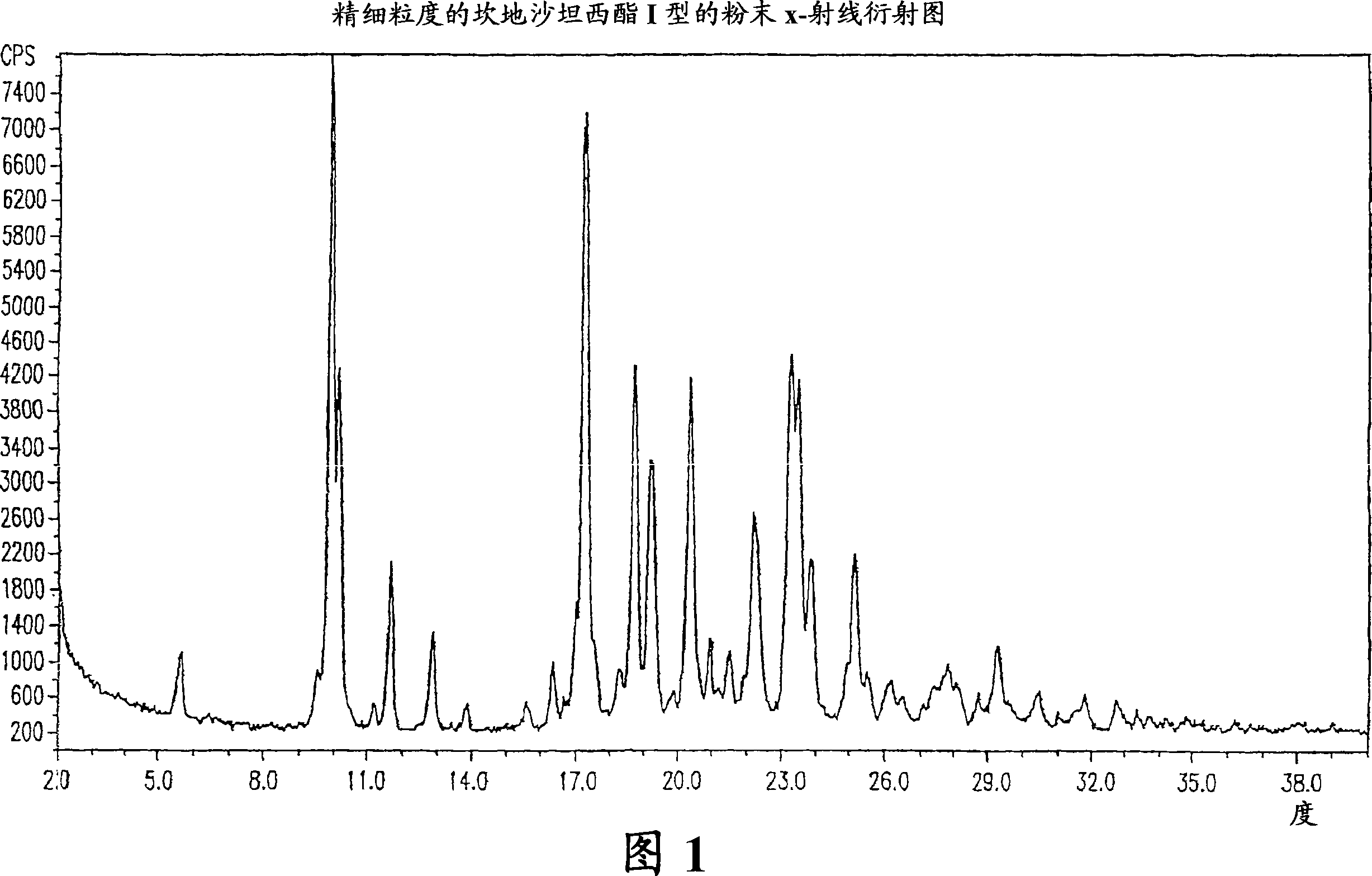
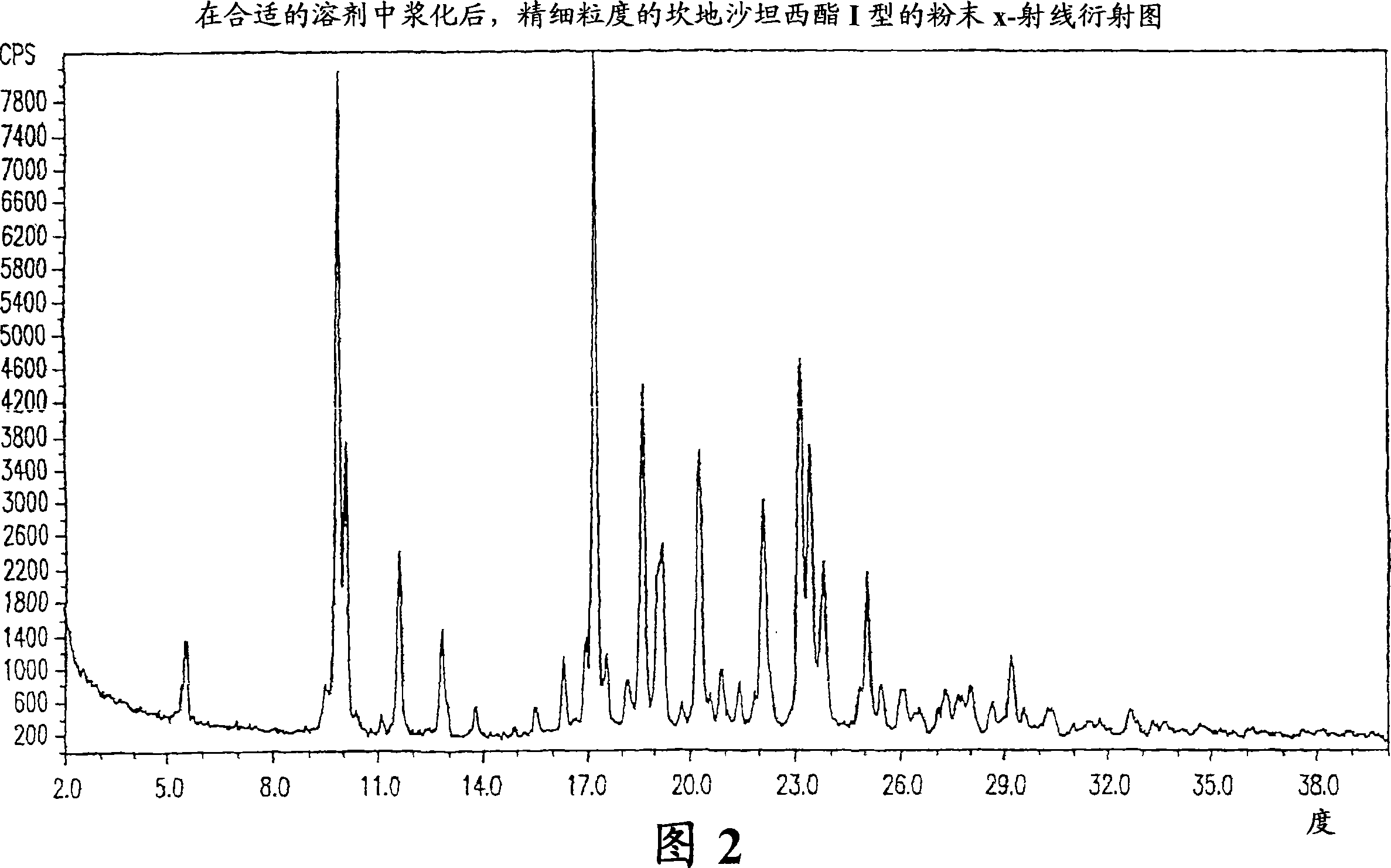
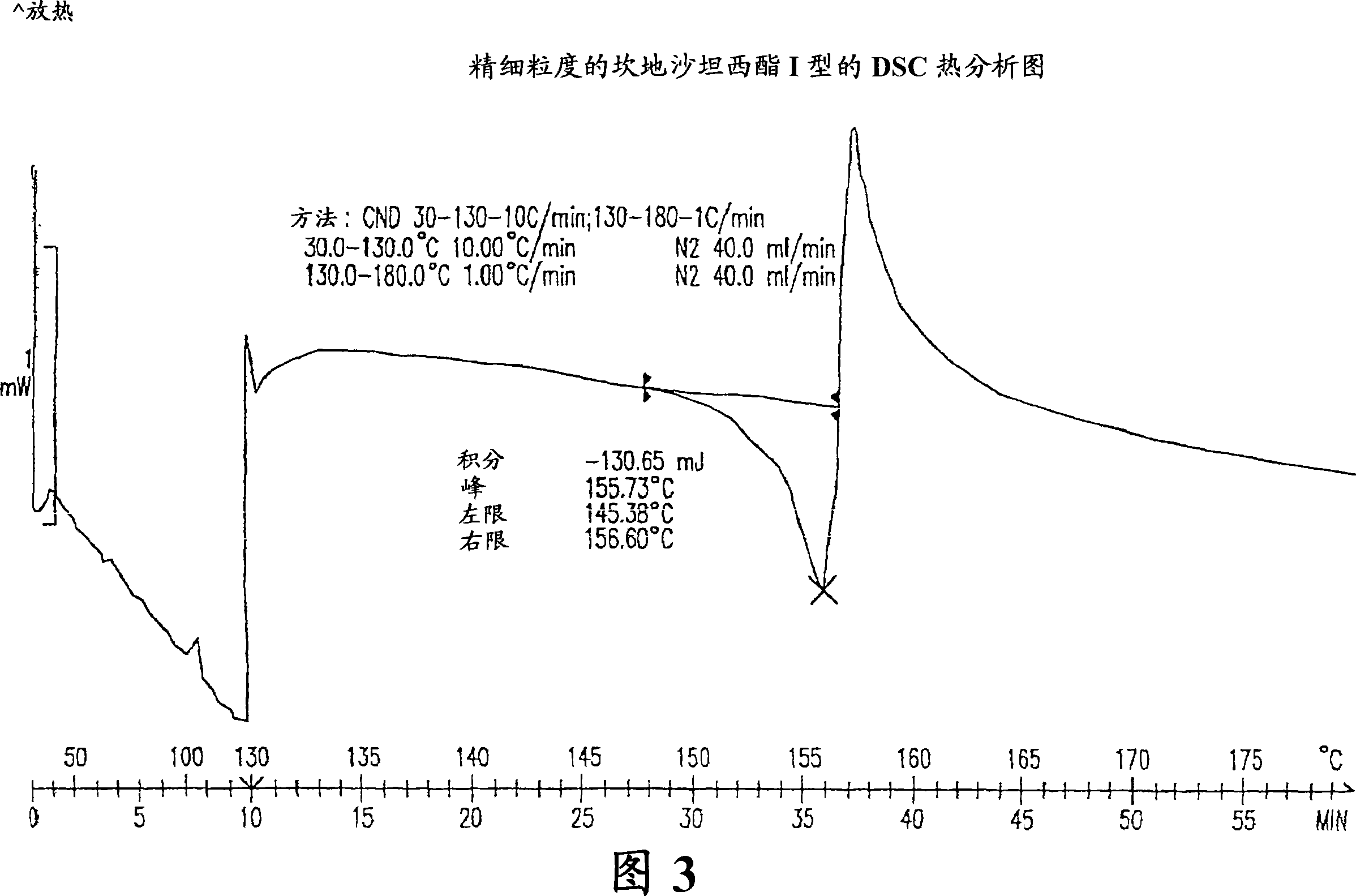
Stable micronized candesartan cilexetil and methods for preparing thereof
A technology of candesartan cilexetil and candesartan, which is applied in the field of stable micronized candesartan cilexetil and its preparation, can solve the problems of candesartan cilexetil particle size reduction and unfavorable chemical stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention also includes a process for the preparation of fine particle size stable candesartan cilexetil. The method includes:
[0030] a) provide fine-grained candesartan cilexetil samples;
[0031] b) in at least one C 1 -C 4 Slurrying the sample in alcohol for about 16 to about 48 hours;
[0032] c) recovery of fine particle size stable candesartan cilexetil.
[0033] Preferably, the candesartan cilexetil obtained by the method is crystal form I.
[0034] preferred, C 1 -C 4 Alcohol is methanol or ethanol. The slurrying in step b) is preferably carried out at a temperature of at least about 15°C, more preferably between about 15°C and about 50°C, most preferably between about 25°C and about 35°C. Preferably, the samples are slurried for about 20 to about 30 hours.
[0035] The fine particles can be recovered by any method known in the art such as cooling the sample; filtering off the solvent; washing the particles, preferably with the solvent add...
Embodiment 1
[0065] Embodiment 1: the preparation of candesartan cilexetil
[0066] Trityl candesartan cilexetil (TCS, 1000g, 1172mmol), toluene (3000mL), methanol (6000mL) and water (50mL) solution was refluxed for about 3-4 hours (HPLC control), under reduced pressure in The solvent was evaporated at 50°C to give a thick oily residue. The residue was dissolved in a toluene / methanol mixture (2960 g, 95:5, w / w) at 50°C. The mixture was then cooled to (-5)°C to (5)°C and maintained at this temperature for about 12 hours. The solid precipitate was filtered off, washed on the filter with cold toluene (1000 mL), then dried under reduced pressure at 60 °C to afford crude candesartan cilexetil Form I (~600 g, L.O.D=17%).
Embodiment 2
[0067] Embodiment 2: the preparation of candesartan cilexetil I type
[0068] 601 g of crude CNS with LOD C for 20-30 hours. The precipitated solid was filtered off and washed with cold absolute ethanol (550 mL) to obtain 644 g of wet matter (LOD=30-40%-80%), then 429 g was dried at 60°C under reduced pressure to obtain candesartancil Ester Form I (-274.5 g, L.O.D. = 0.12%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com