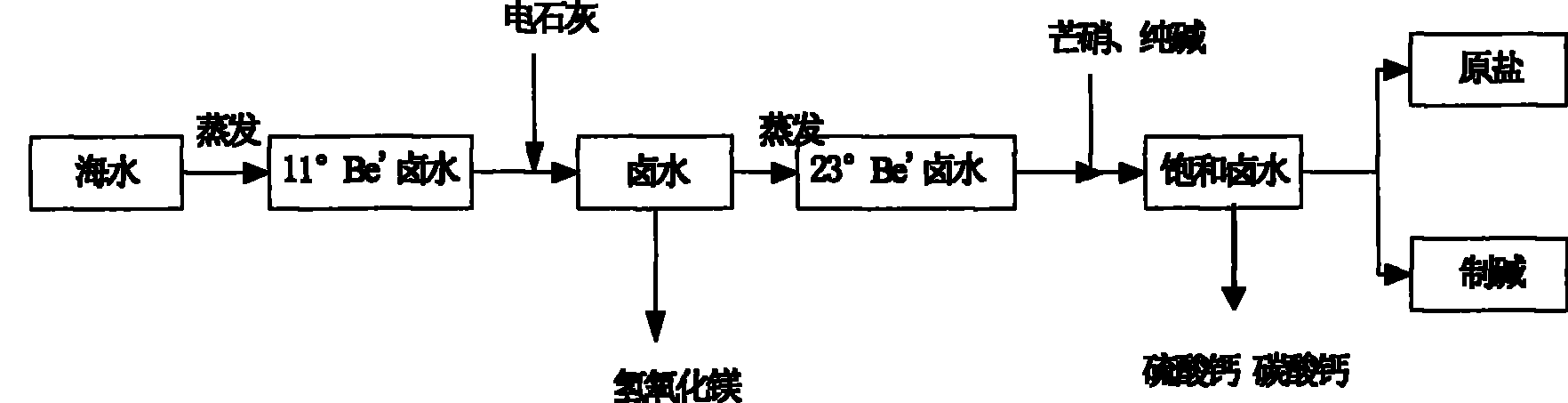
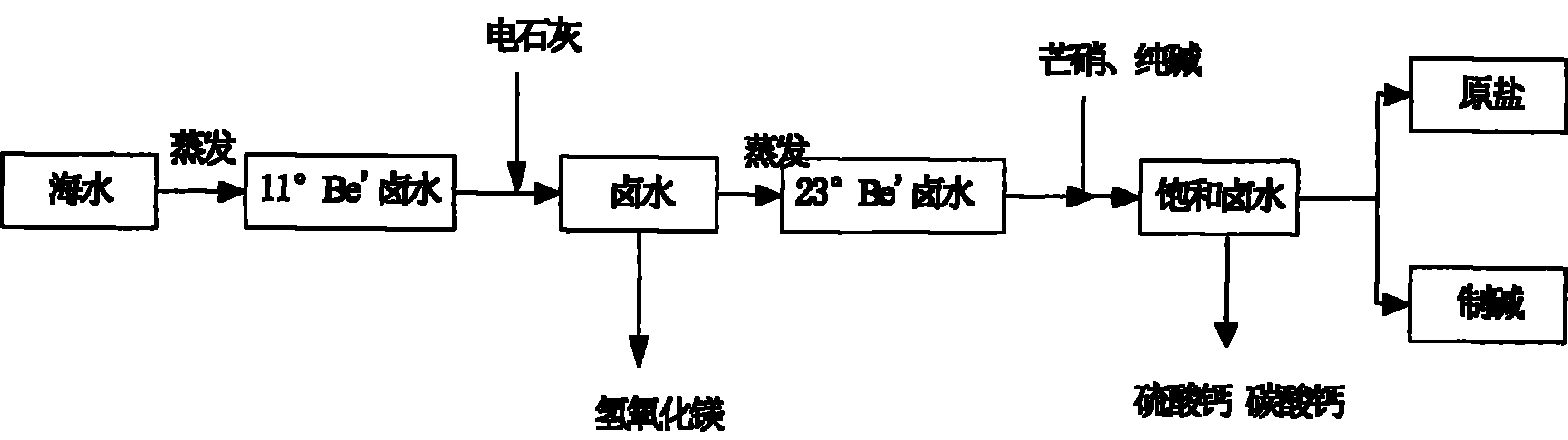
Method for removing magnesium ion and calcium ion in middle-degree brine in salt-field
A technology of magnesium ions and calcium ions, applied in the direction of alkali metal chlorides, etc., can solve the problems of reduced brine evaporation intensity, low halogenation and salt production, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Select the waste slag carbide lime produced by chlor-alkali plant as the basic raw material to remove Mg in brine 2+ , so that Mg 2+ It is precipitated in the form of magnesium hydroxide, and the carbide lime residue after the production of acetylene gas in Tianjin Dagu Chemical Plant is selected. The initial concentration of brine is 11°Be / , and its specific operation is as follows:
[0023] 1) Preparation of Ca(OH)2 gray milk
[0024] In a continuous settling tank with stirring, the ratio of calcium carbide and brine is 1:3 by weight, in the lime milk, Ca(OH) 2 The content is 40g / l, and the ash is separated and discharged in the settling tank at the same time.
[0025] 2) Remove Mg 2+
[0026] According to Mg in brine 2+ content, calculate the amount of milk of electric lime, and control the excessive amount of milk of electric lime within 5%. Using a continuous sedimentation reaction tank, slowly add the emulsified calcium carbide milk into the brine, and when...
Embodiment 2
[0030] The carbide lime residue after the production of acetylene gas in Tianjin Dagu Chemical Plant was selected. The initial concentration of brine is 13.6 ° Be ', and its specific operation is as follows:
[0031] 1) Ca(OH) 2 Gray milk preparation
[0032] In a continuous settling tank (emulsification tank) with agitation, the ratio of calcium carbide and brine is 1:4 by weight, and in the gray milk
[0033] Ca(OH) 2 The content is 30g / l, and the ash is separated and discharged in the settling tank at the same time.
[0034] 2) Remove Mg 2+
[0035] According to Mg in brine 2+ content, calculate the amount of milk of electric lime, and control the excessive amount of milk of electric lime within 5%. Using a continuous sedimentation reaction tank, slowly add the emulsified milk of calcium carbide into the brine, and when the pH is controlled to 11.8, the Mg 2+ with OH - Can be fully combined to form an insoluble substance Mg(OH) 2 , Mg in brine 2+ The content is 0. ...
Embodiment 3
[0039] The carbide lime residue after the production of acetylene gas in Tianjin Dagu Chemical Plant was selected. The initial concentration of brine is 18 ° Be ', and its specific operation is as follows:
[0040] 2) Ca(OH) 2 Gray milk preparation
[0041] In a continuous emulsification tank with agitation, the ratio of calcium carbide and brine is 1:2.5 by weight, and Ca(OH) in lime milk 2 The content is 60g / l, and the ash is separated and discharged in the settling tank at the same time.
[0042] 2) Remove Mg 2+
[0043] According to Mg in brine 2+ content, calculate the amount of milk of electric lime, and control the excessive amount of milk of electric lime within 10%. Using a continuous sedimentation reaction tank, slowly add the emulsified calcium carbide milk into the brine, and when the pH is controlled to 12.5, the Mg 2+ with OH - Can be fully combined to form an insoluble substance Mg(OH) 2 , Mg in brine 2+ The content is 0. Mg(OH) 2 It is separated fro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com