Method for removing magnesium ion and calcium ion in middle-degree brine in salt-field
A technology of calcium ion and magnesium ion, which is applied in the direction of alkali metal chloride, etc., can solve the problems of low brine formation and salt production, and reduced brine evaporation intensity, so as to improve salt quality, reduce crystallization area, and reduce evaporation resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
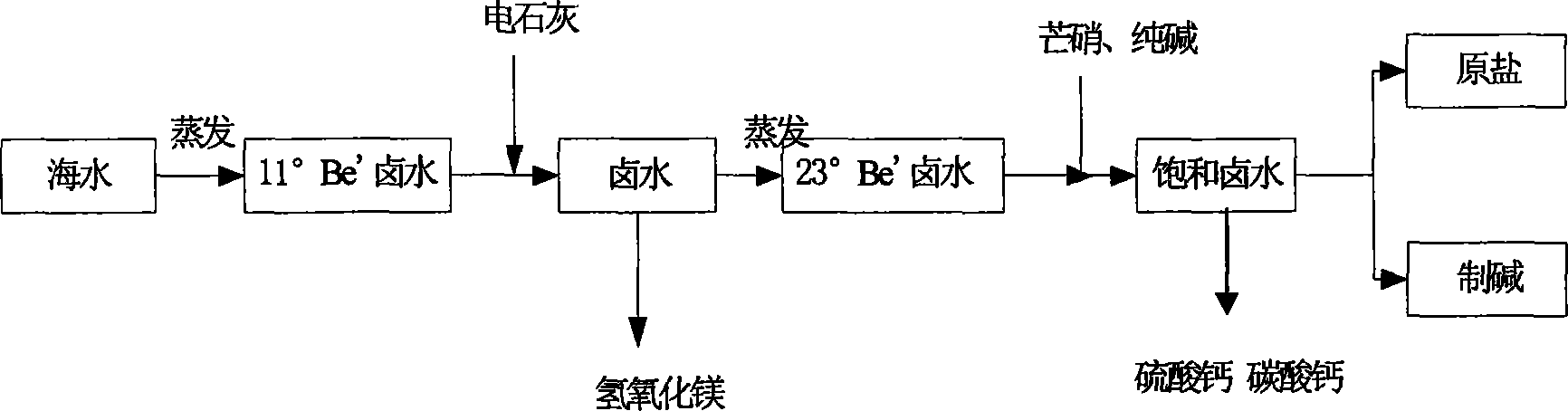
[0022] Select the waste slag electrolime produced by the chlor-alkali plant as the basic raw material to remove Mg in the brine 2+ , making Mg 2+ It is precipitated in the form of magnesium hydroxide, and the electric lime waste residue after the production of acetylene gas in Tianjin Dagu Chemical Plant is selected. The initial concentration of brine is 11°Be', and its specific operation is as follows:
[0023] 1) Preparation of Ca(OH)2 gray milk
[0024] In a continuous settling tank with stirring, the ratio of electric lime to brine is 1:3 by weight. In the ash milk, Ca(OH) 2 The content is 40g / l, and the ash and slag are separated and discharged in the settling tank at the same time.
[0025] 2) Remove Mg 2+
[0026] According to Mg in brine 2+ content, calculate the amount of electric lime milk, and the excessive amount of electric lime milk is controlled within 5%. A continuous sedimentation reaction tank was used, and the emulsified electric lime milk was slowly ...
Embodiment 2
[0030] The electric lime waste residue after the production of acetylene gas in Tianjin Dagu Chemical Plant is selected. The initial concentration of brine is 13.6°Be', and its specific operation is as follows:
[0031] 1) Ca(OH) 2 Preparation of grey milk
[0032] In a continuous settling tank (emulsification tank) with stirring, the ratio of electric lime and brine is 1:4 by weight, and Ca(OH) in the lime milk 2 The content is 30g / l, and the ash and slag are separated and discharged in the settling tank at the same time.
[0033] 2) Remove Mg 2+
[0034] According to Mg in brine 2+ content, calculate the amount of electric lime milk, and the excessive amount of electric lime milk is controlled within 5%. Using a continuous sedimentation reaction tank, the emulsified electric lime milk was slowly added to the brine, and when the pH was controlled to 11.8, the Mg 2+ with OH - Can fully combine to form insoluble substance Mg(OH) 2 , Mg in brine 2+ The content is 0. Mg(...
Embodiment 3
[0038] The electric lime waste residue after the production of acetylene gas in Tianjin Dagu Chemical Plant is selected. The initial concentration of brine is 18°Be', and its specific operation is as follows:
[0039] 2) Ca(OH) 2 Preparation of grey milk
[0040] In a continuous emulsification barrel with stirring, the ratio of electric lime and brine is 1:2.5 by weight, and Ca(OH) in the lime milk 2 The content is 60g / l, and the ash and slag are separated and discharged in the settling tank at the same time.
[0041] 2) Remove Mg 2+
[0042] According to Mg in brine 2+ content, calculate the amount of electric lime milk, and the excessive amount of electric lime milk is controlled within 10%. Using a continuous sedimentation reaction tank, the emulsified electric lime milk was slowly added to the brine, and when the pH was controlled to 12.5, the Mg 2+ with OH - Can fully combine to form insoluble substance Mg(OH) 2 , Mg in brine 2+ The content is 0. Mg(OH) 2 It i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com