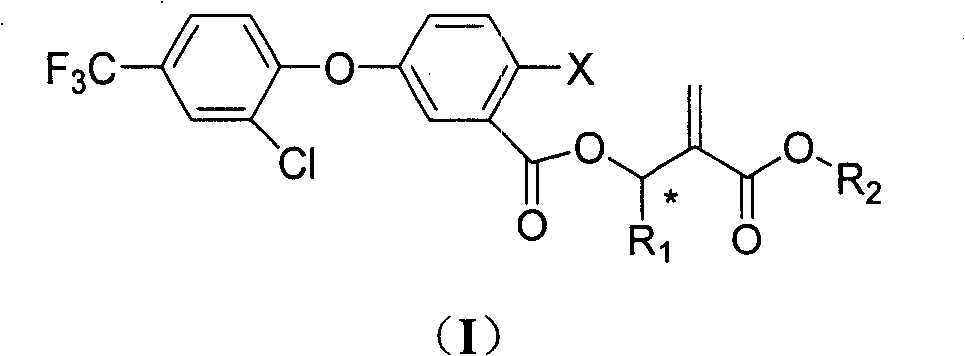
Benzoate compounds and uses thereof
A technology of ester compounds and benzoic acid, which is applied in the fields of application, organic chemistry, animal repellent, etc., and can solve the problems of undisclosed alkyl acrylate compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of compound 1:
[0041]
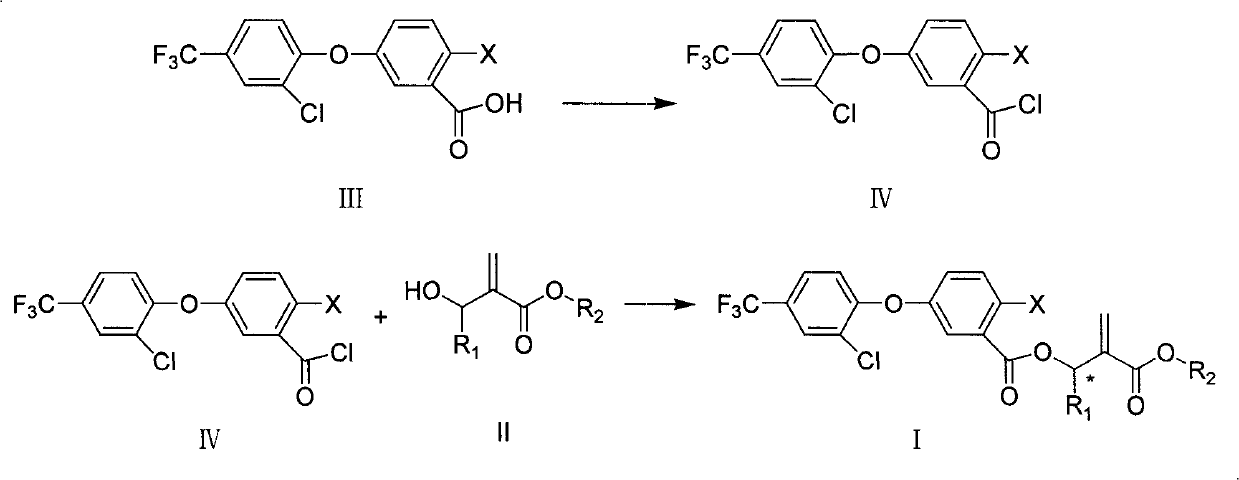
[0042] Add III-1 (11.6 grams, 0.032 moles, commercially available) and 150 milliliters of methylene chloride in 250 milliliters of reaction bottles, stir, add oxalyl chloride (6.1 grams, 0.048 moles), 2 drops of dimethylformamide , stirred at room temperature for 5 hours, and concentrated to obtain 11.0 g of acid chloride, which appeared as a yellow liquid (IV-1).
[0043]
[0044] Add II-1 ((0.39 g, 3.0 mmol), triethylamine (0.46 g), 5 ml of dichloromethane in a 50 ml reaction flask, add IV-1 (1.2 g, 3.2 mmol) dropwise under stirring and cooling ) and 10 milliliters of dichloromethane, added in about half an hour, and reacted for 6 hours at room temperature. After the reaction was completed, 100 milliliters of dichloromethane and 50 milliliters of water were added to the reaction solution for liquid separation, and the dichloromethane layer was used successively Wash with 100 ml of saturated aqueous sodium bi...
Embodiment 2
[0045] Embodiment 2: the synthesis of compound 4:
[0046]
[0047] Add III-1 (0.37 g, 1.0 mmol, commercially available) V-4 (0.2 g, 1.0 mmol) in a 50 ml reaction flask, 0.2 g of potassium carbonate, 10 ml of dimethylformamide, 40 °C and stirred for 1 hour. After completion of the reaction, add 100 milliliters of dichloromethane and 50 milliliters of water to the reaction solution, separate the layers, wash with water, dry the oil layer with anhydrous magnesium sulfate, concentrate, and use a silica gel column whose developing solvent is ethyl acetate:petroleum ether=1:3 After separation by chromatography, 0.38 g of light yellow oil (compound 4) was obtained.
[0048] Other compounds in Table 1 can be prepared by similar methods above.
[0049] Biotest example
[0050] Dissolve the original drug with acetone, and add it to a certain amount of water containing 0.1% Tween 80 according to the designed dose in Table 2 below to prepare a test solution of the compound at a cer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com