Novel shuttle vector
一种穿梭载体、表达载体的技术,应用在抗肿瘤剂领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] In the preparation of liquid preparations suitable for oral administration, sugars such as water, sucrose, sorbitol, and fructose; alcohols such as polyethylene glycol and propylene glycol; oils such as sesame oil, olive oil, and soybean oil can be used; Additives for preparations such as p-hydroxybenzoate and other preservatives. In addition, in the manufacture of solid preparations such as capsules, tablets, powders, or granules, for example, excipients such as lactose, glucose, sucrose, and mannose; disintegrants such as starch and sodium alginate; stearic acid Lubricants such as magnesium and talc; Binders such as polyvinyl alcohol, hydroxypropyl cellulose and gelatin; Surfactants such as fatty acid esters; Plasticizers such as glycerin.
[0062] Among preparations suitable for parenteral administration, preparations for intravascular administration such as injections and spot drops can be preferably prepared using an aqueous solvent that is isotonic with human bloo...
Embodiment 1
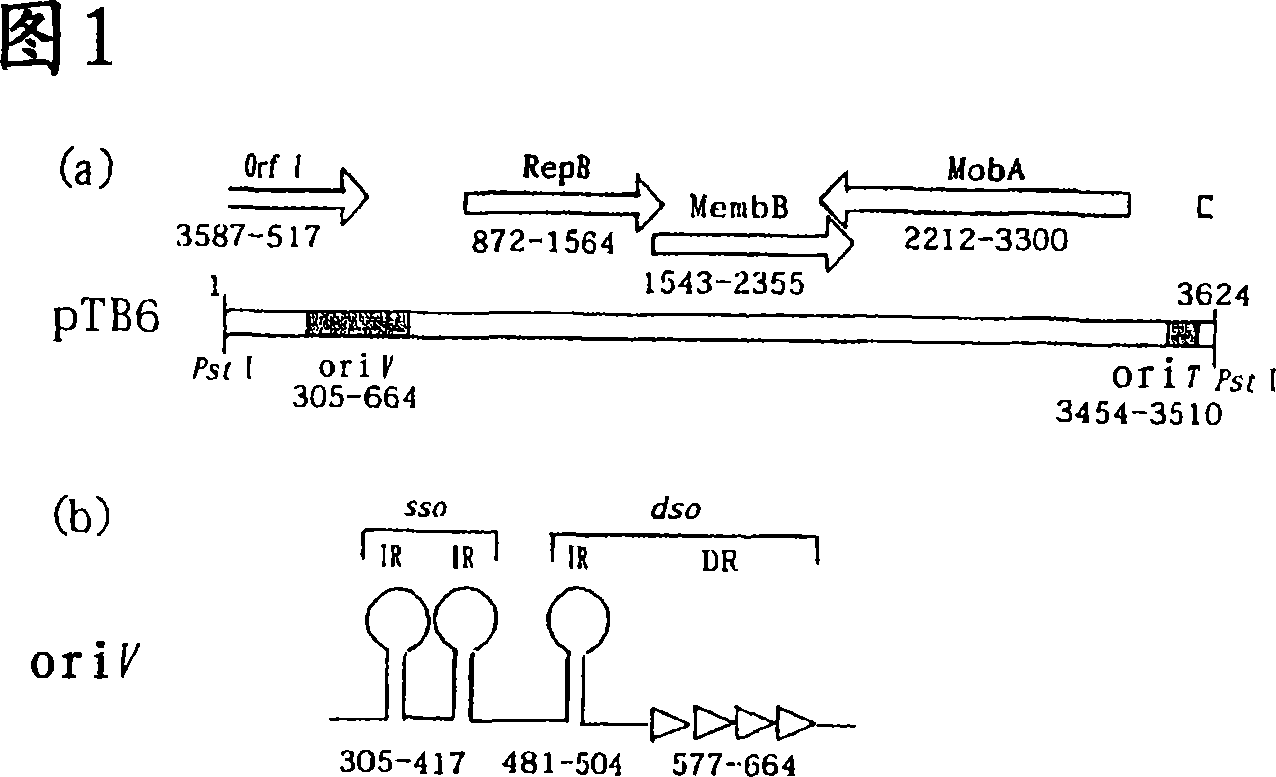
[0069] (Structure Analysis of Plasmid pTB6)
[0070] [Materials and methods]
[0071] 1. Strains, plasmids and media
[0072] The bacterial strains and plasmids used in the present invention are listed in Table 1. In LB broth medium (10 g of Bacto-tryptone), 5 g of yeast extract, 5 g of NaCl and 0.1% glucose / liter), Escherichia coli was cultured aerobically at 37° C. Colonies formed in broth. Supplemented 50mM sucrose, 0.34% cysteine, 0.02% sodium ascorbate MRS broth medium (manufactured by Difco Laboratories, U.S.), cultivated Bifidobacterium longum 105-A anaerobically at 37°C (referring to non-patent Literature 12). Antibiotics (50 μg / ml ampicillin (Ap) and / or 75 μg / ml spectinomycin (sp)) were added as needed. According to the method described in Non-Patent Document 12, colonies were formed on a medium containing 1.5% agar using a Gas-Pak anaerobic system (manufactured by BBL, USA).
[0073] [Table 1]
[0074] strain or plasmid
relevant features
Liter...
Embodiment 2
[0110] (Construction of Shuttle Vector pAV001)
[0111] [Construction of plasmid]
[0112] From pBLES100, the sequence containing spectinomycin adenyltransferase (AAD box) derived from Enterococcus faecalis (Enterococcus faecalis) was amplified by PCR, subcloned into pCR-BluntII-TOPO vector (manufactured by Invitrogen), and pCRTOPO- ScaI-AAD-Eaml105I. In addition, ScaI was added to the forward primer, and an Eaml105I restriction enzyme site was added to the reverse primer.
[0113] As shown in Figure 5, the cloning vector pGFPuv purchased from Invitrogen (DEFINITION: Cloning vector pGFPuv. Accession number: U62636 version: U62636.1 GI: 1490528) consists of the GFPuv gene and the multiple cloning sites (Multi-Cloning Site , MCS), ampicillin resistance gene, OriC (pUC Ori) composition of E. coli plasmid replication origin.
[0114] The ampicillin resistance gene locus of this pGFPuv was cleaved with restriction enzymes Eaml105I and ScaI to prepare a removed long fragment. Si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com