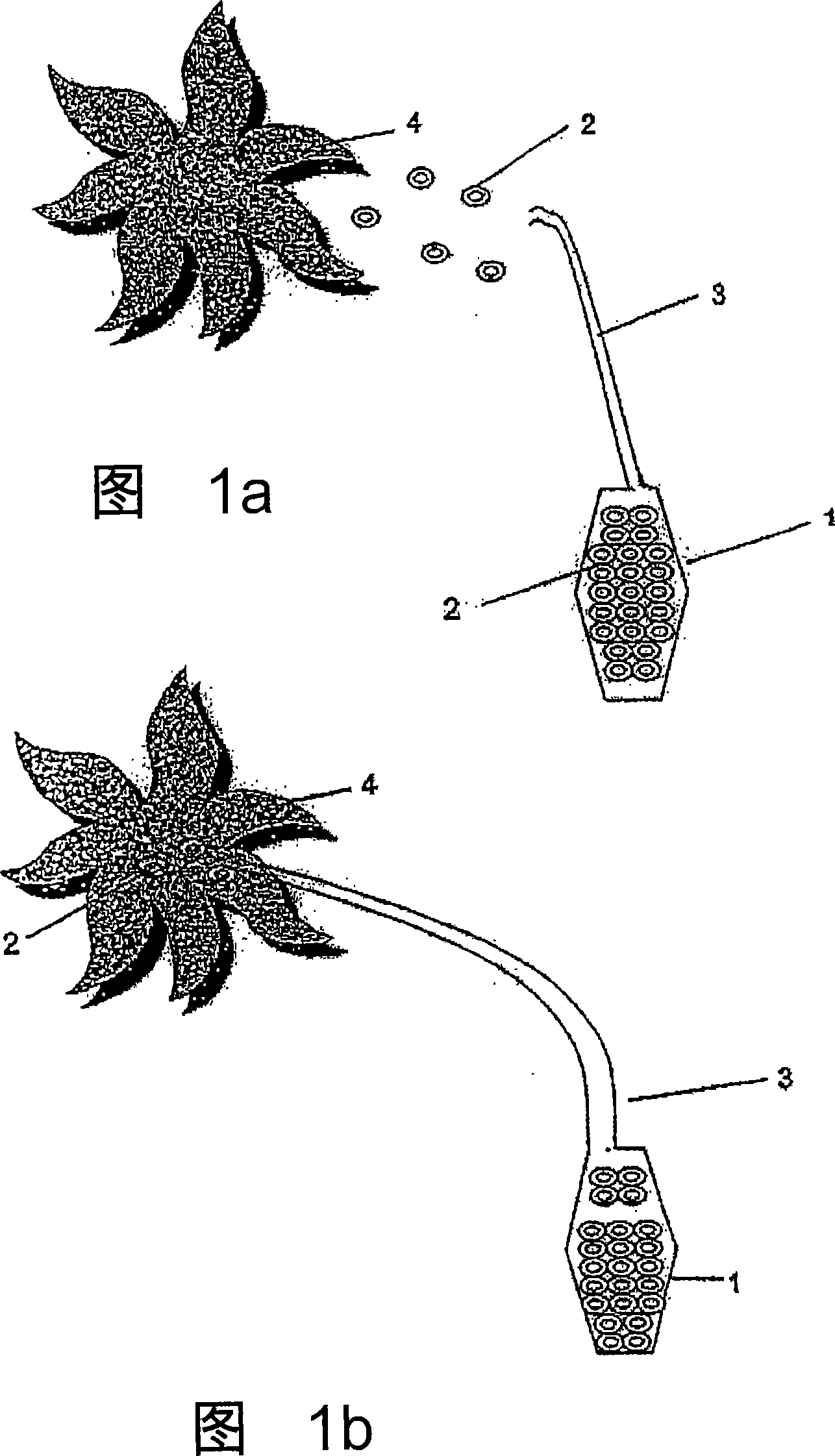
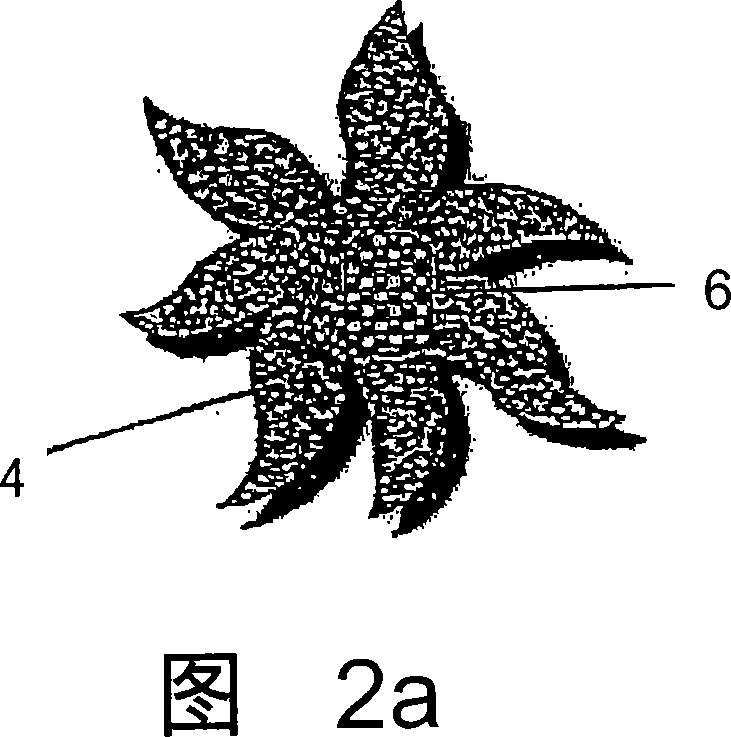
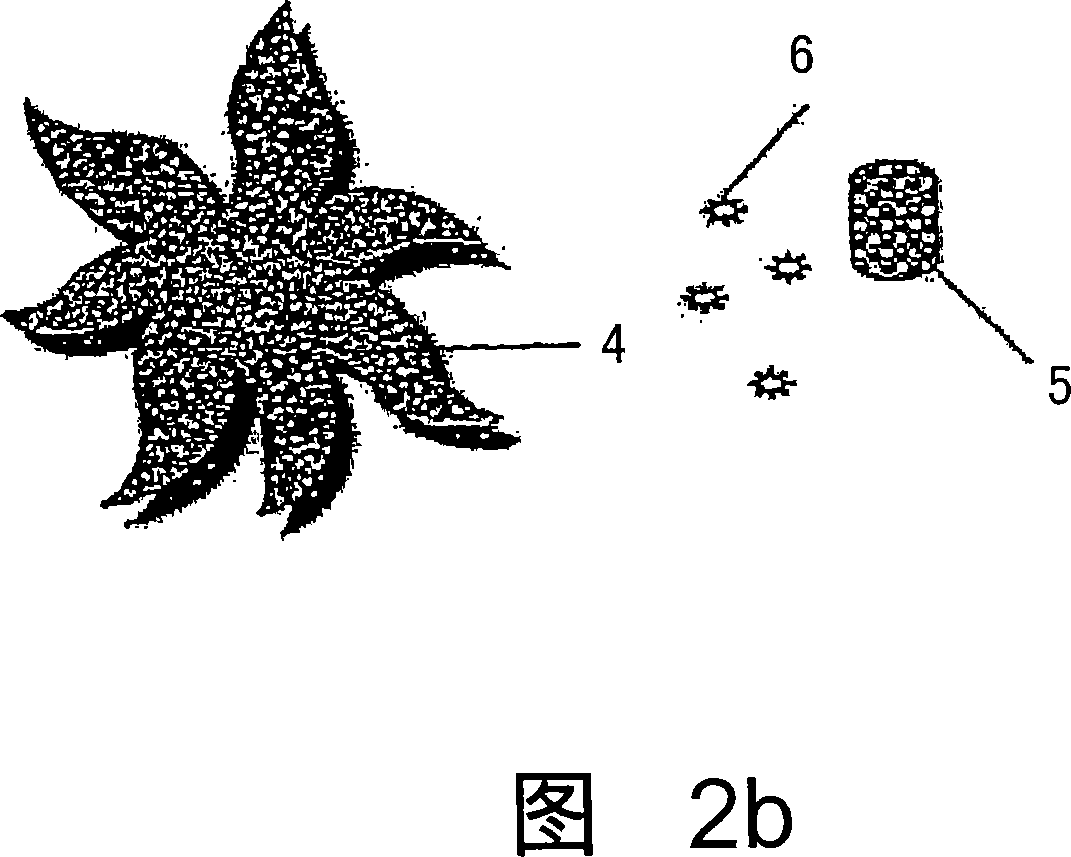
Controlled and directed local delivery of anti-inflammatory compositions
A composition, topical technology, applied in the field of pain reduction or elimination system, which can solve the problems of insufficient filling of patients' needs, prolonged drug action time, limited drug amount, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Randomly assign four animals per group with CCI "neuropathic pain". CCI animals are subjected to a series of behavioral tests (ie mechanical tactile allodynia and thermal nociception tests) following administration of test compounds by systemic injection or local delivery via Alzet(R) osmotic pumps. The first dose was given prior to the test and subsequent doses were given at the half-life of each compound.
[0083] Behavioral tests: Von Frey test; hot plate test
[0084] Compounds of interest were delivered locally by osmotic pump, and up to 8 behavioral trials (4 per behavior), including the baseline trial, were performed. The study duration was 22 days or less. Following systemic or topical administration, behavioral assays are performed as described below to determine the optimal dosing regimen for any proposed compound of interest that may be effective in the methods of the invention.
[0085] The activity of compounds was evaluated using an in vivo chron...
Embodiment 2
[0094] Comparison and assessment of protein-based inhibitors of TNFα function in a rat model of chronic constriction injury (CCI): systemic versus local delivery
[0095] On the day of the start of surgery, a systemic dose of the compound is given by subcutaneous injection, and then the dosing period is determined by the half-life of the compound. The initial dose level of the compound should be administered by repeated injections. Local administration of the compounds is achieved by constant local infusion through implanted osmotic pumps.
[0096]Behavioral tests: Von Frey filament test (days 7, 14 and 21), thermal hyperalgesia test (days 8, 16 and 22).
[0097] therapy group
number of animals
systemic administration
Topical administration
Carrier (CCI only)
7
7
Gabapentin (positive control)
7
7
Compound 1
7
7
Compound 2
7
7
Compound 3
7
7
total
...
Embodiment 3
[0102] PLGA 50:50 / rhBMP-2 microsphere preparation
[0103] Dichloromethane (Aldrich MO 02249E0, D=1.325) was used as solvent. PLGA 50 / 50 was obtained from Sigma (Lactel BP-0100, Lot 56H1176). Recombinant human bone morphogenetic protein (rhBMP) (7.31 mg / vial) was prepared in the laboratory according to methods previously described and known to those skilled in the art. Dissolve 1 vial of rhBMP in 1 ml sterile water (preferably filter sterilized). PLGA (513.4 mg) was dissolved in 8 mL of dichloromethane.
[0104] The rhBMP was first dissolved in sterile water, and then the aqueous BMP solution was emulsified in the PLGA polymer solution. Briefly, combine 0.5 mL of BMP solution and 4 mL of PLGA / MeCl 2 , emulsify with a homogenizer (medium setting) for 45 seconds. Transfer the emulsion into a syringe with an 18-gauge needle. Add 60 ml of 3% PVA to a 150 ml glass beaker. The homogenizer was set at 3, the 3% PVA solution was stirred, and the emulsified polymer / BMP solution w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com