Preparation method of acetylsalicylic acid and chitin-2-6-bit graft
A technology of acetylsalicylic acid and chitosan, applied in the direction of sugar derivatives, sugar derivatives, esterified saccharides, etc., can solve problems such as gastric perforation, inability to take internally directly, strong irritation of salicylic acid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
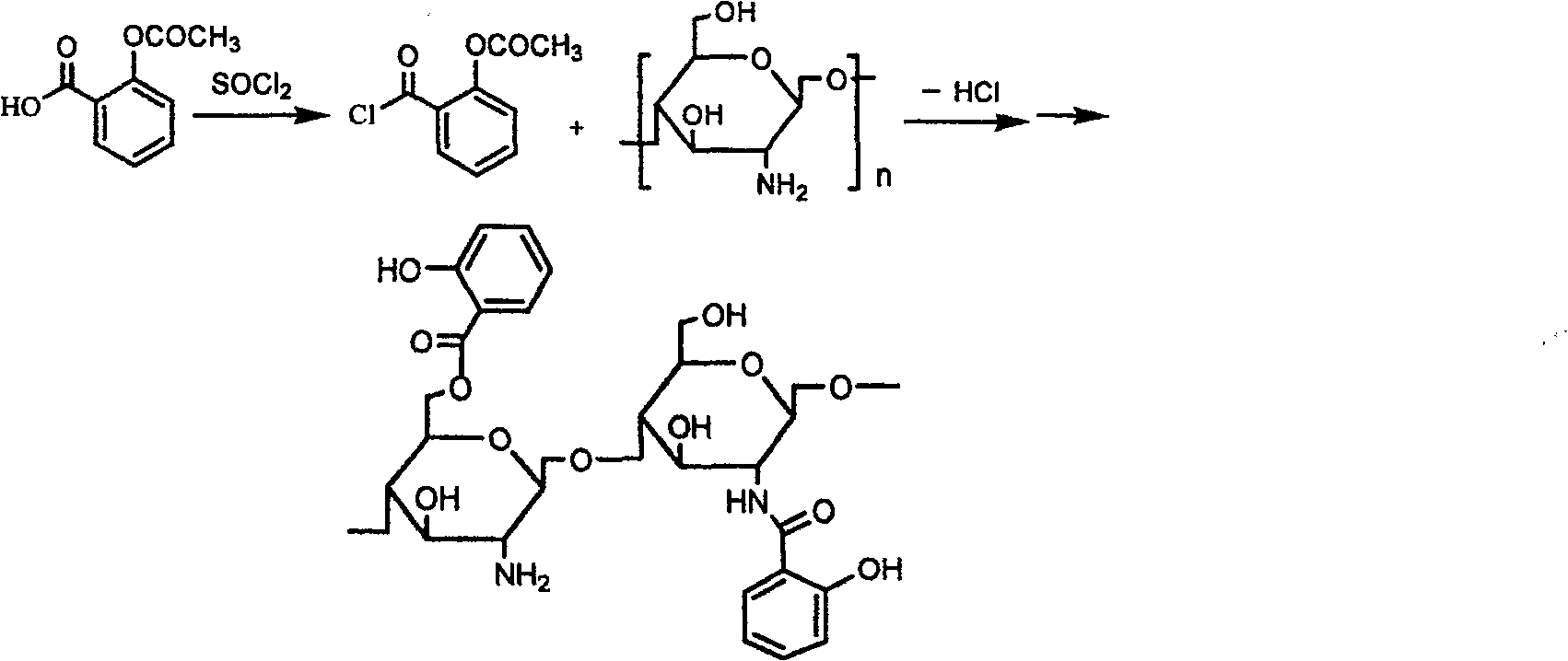
[0021] Example 1. Add an appropriate amount of acetylsalicylic acid into the reaction flask, slowly dropwise add freshly distilled thionyl chloride (added at a molar ratio of 1:1), and stir under reflux for 3 hours to obtain A solution.
[0022] Swell an appropriate amount of chitosan with methanesulfonic acid, slowly add equimolar (chitosan is calculated as monosaccharide unit) A solution dropwise, stir and react at 20° C. for 2 hours, and let stand. Add a large amount of acetone, separate out the precipitate, neutralize the precipitate with ammonia water, wash with water, wash with ethanol, and spray dry or freeze dry the solid to obtain a derivative mainly grafted at the 6-position, with an average grafting rate of about 60%.
Embodiment 2
[0023] Example 2: Add chitosan / acetylsalicylic acid with a weight-feeding ratio of 6:5 to a reaction flask, add thionyl chloride solution dropwise, and reflux and stir for 3 hours to obtain A solution.
[0024] Put chitosan with chitosan / acetylsalicylic acid weight ratio of 6:5 into 15 times the amount of dioxane or DMF, add p-toluenesulfonic acid to swell, and react with solution A at 20°C 2hr, stand still. Add a large amount of ammonia water to neutralize, let stand, and centrifuge. The precipitate is washed with water and ethanol, and the solid is spray-dried or freeze-dried to obtain a derivative mainly grafted at the 6-position, with an average grafting rate of about 50%.
Embodiment 3
[0025] Example 3: Add chitosan / acetylsalicylic acid with a weight-feeding ratio of 6:5 to a reaction flask, add thionyl chloride solution dropwise, and reflux and stir for 3 hours to obtain A solution.
[0026] Add chitosan with chitosan / acetylsalicylic acid weight ratio of 6:5 into the reaction flask, use dimethyl sulfoxide or DMF as solvent, and add a little pyridine or triethylamine. Slowly drop the A solution into the reaction bottle, keep the temperature at 20°C, stir for 3 hours, and let stand. Filter, wash the product with ethanol, and centrifuge to obtain 2-position and 6-position mixed graft derivatives, with an average grafting rate of about 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com