Desulfurization for simultaneous removal of hydrogen sulfide and sulfur dioxide
一种二氧化硫、硫化氢的技术,应用在化学仪器和方法、分离方法、弥散粒子分离等方向,能够解决环境问题、化学品过度损失、催化剂活性丧失等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
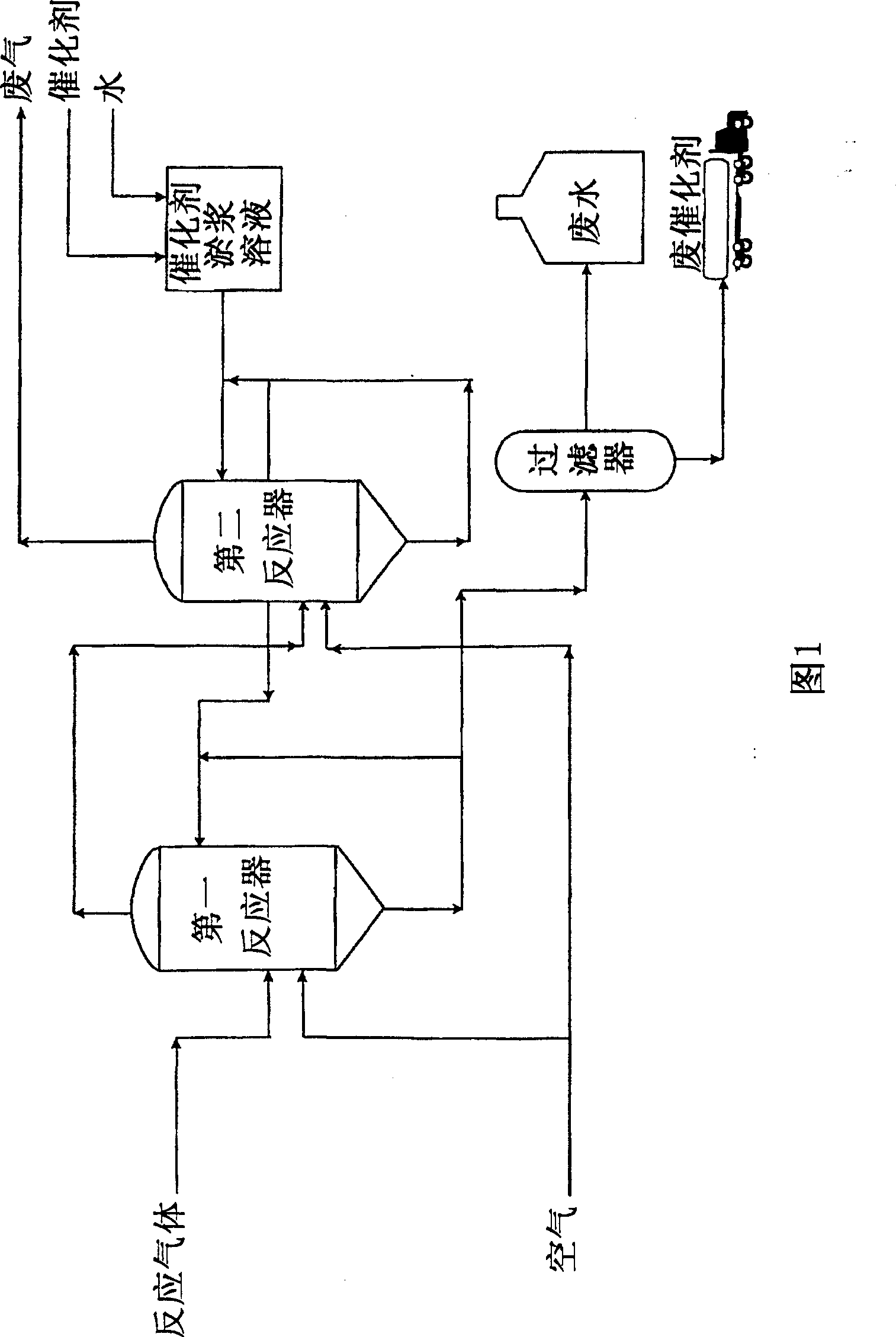
Image
Examples
Embodiment 1
[0050] Example 1 illustrates the effect of simultaneous removal of hydrogen sulfide and sulfur dioxide in a batch wet oxidation reaction without the use of a catalyst.
[0051] 1.5 liters of water were introduced into a stirred reactor, wherein hydrogen sulfide, sulfur dioxide and air were introduced through a porous diffuser positioned at the bottom of the reactor so that the flow of hydrogen sulfide was 10ml / min, and the flow of sulfur dioxide was 5ml / min, as the oxidant The flow rate of air is 100ml / min. After the reaction, the product gas was analyzed by gas chromatography, and the result was converted into removal efficiency using the following equation.
[0052]
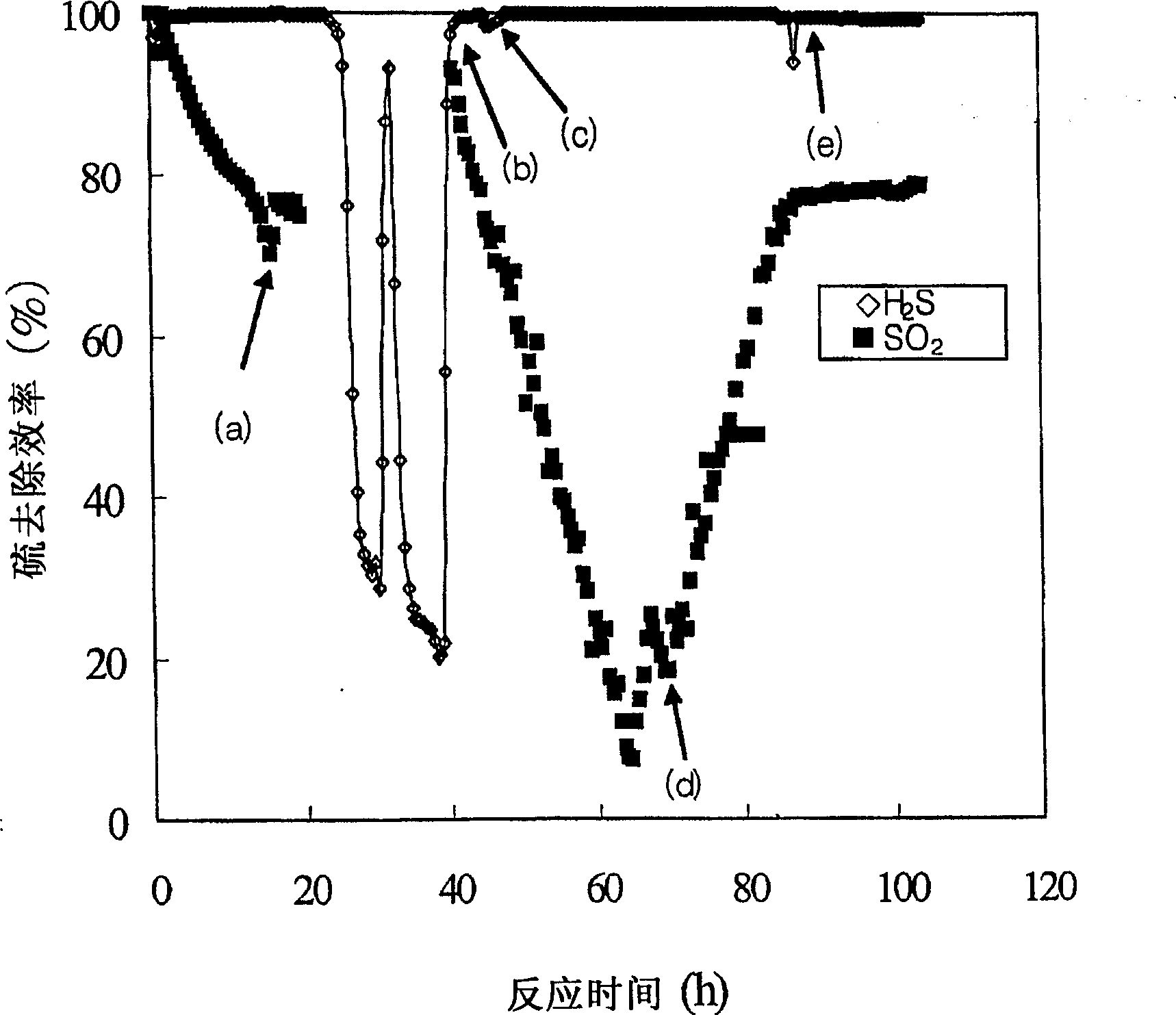
[0053] figure 2 In represents the sulfur removal efficiency as a function of reaction time. exist figure 2 Among them, (a), (b), (c), (d) and (e) represent respectively when SO 2 Close, SO 2 Open, H 2 S off, H 2 S opens and the point when the oxidizing agent becomes oxygen.
[0054] Such as figure 2...
Embodiment 2
[0056] The procedure of Example 1 was repeated except that 3 g of 6 wt% Fe / MgO catalyst was used in Example 2. 20g of MgO was dispersed in 200ml of water, and 1N ferric nitrate solution was added thereto so that Fe became 6wt% relative to MgO, and then dried and roasted at 450°C to prepare 6wt% Fe / MgO catalyst.
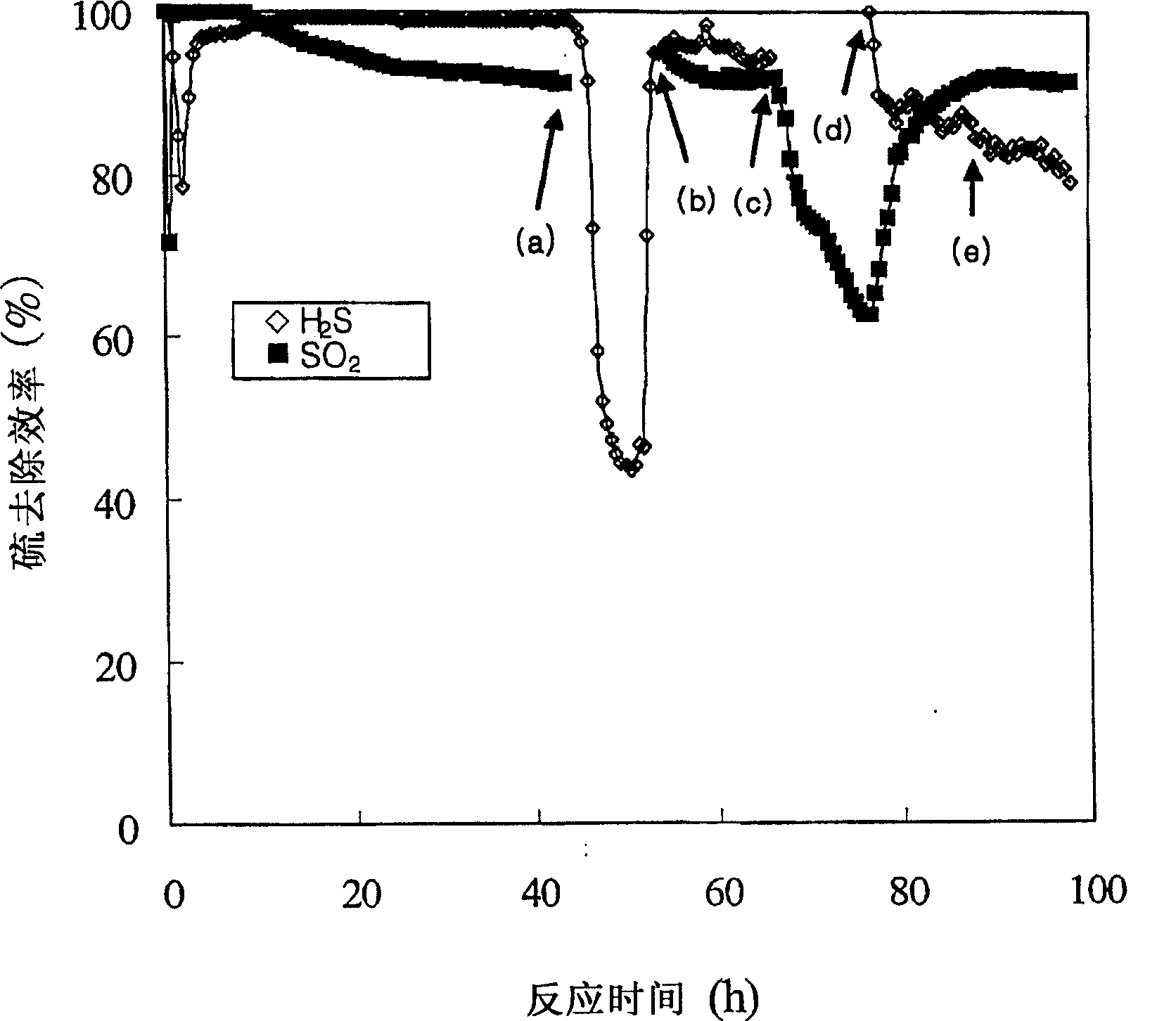
[0057] result in image 3 shown in . exist image 3 Among them, (a), (b), (c), (d) and (e) represent respectively when SO 2 Close, SO 2 Open, H 2 S off, H 2 S opens and the point when the oxidant becomes nitrogen.
[0058] image 3 It shows that the respective removal efficiency and simultaneous removal efficiency of hydrogen sulfide and sulfur dioxide in Example 2 using Fe / MgO catalyst are much higher than that in Example 1 without catalyst. After point (e) when the oxidant is changed to nitrogen, the removal efficiency of hydrogen sulfide and sulfur dioxide gradually decreases.
Embodiment 3
[0060] Using 3 g of 6 wt% Fe / MgO catalyst, except that the reaction gas ratio between hydrogen sulfide and sulfur dioxide was changed to 5:5, 5:15 and 10:5 and except that air was used as oxidant while the total gas flow was fixed at 110 / ml / min, repeat the same desulfurization step as in Example 2.
[0061] As a result, the removal efficiency of hydrogen sulfide is high when the concentration of sulfur dioxide is higher than that of hydrogen sulfide, as Figure 4 shown. This means that sulfur dioxide plays a key role as an oxidant in the desulfurization reaction of the present invention. Specifically, when the ratio between hydrogen sulfide and sulfur dioxide was 5:5 or 10:5, a sudden drop in removal efficiency was observed at the beginning of the reaction, which indicated that there was an induction period in the desulfurization reaction of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com