Method of preparing multiple doses of a pharmaceutical solution from a single-dose
a technology of ranibizumab and single dose, which is applied in the field of preparing multiple individual doses of a pharmaceutical solution, can solve the problems of significant portion of the drug being unused and wasted, and achieve the effect of reducing the cost of treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
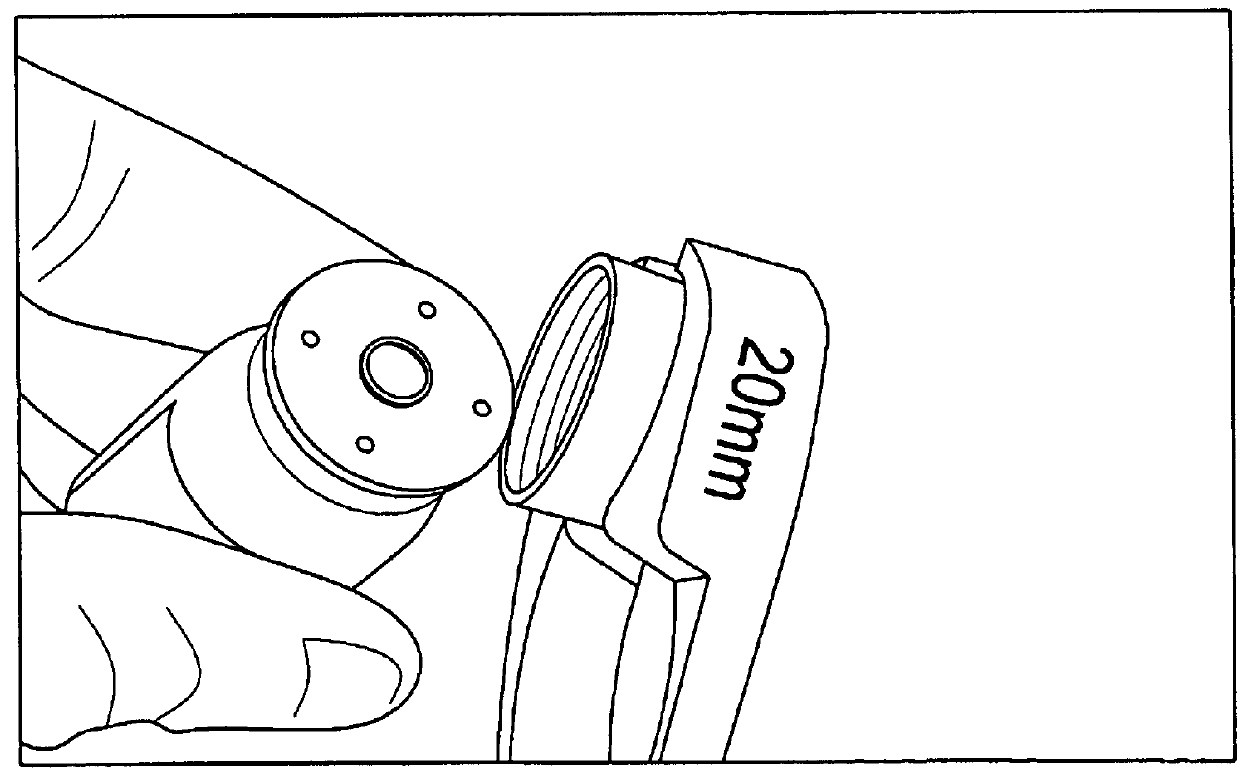
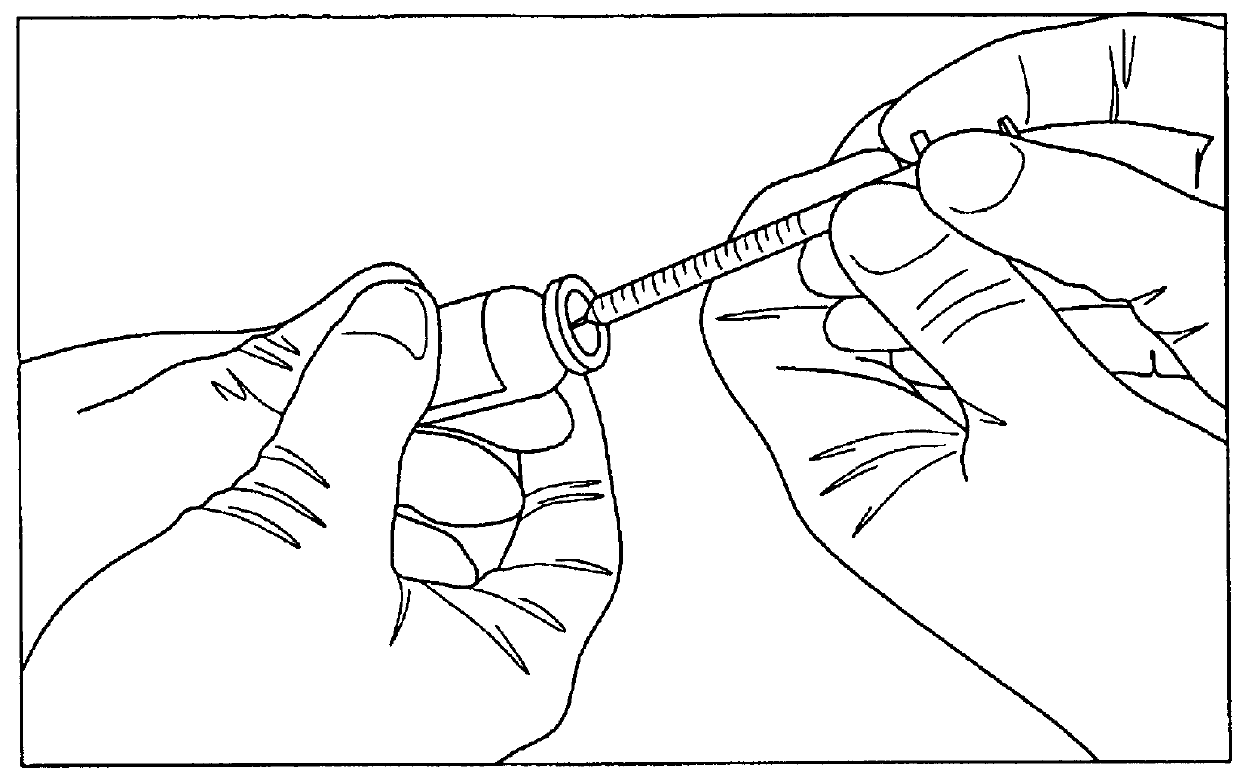
[0017]The present invention is based, in part, on the discovery that due to the dead space in a syringe, particularly a tuberculin syringe, a significant portion of the drug is unused and wasted. In particular, the present inventors discovered that ranibizumab is supplied by the manufacturer in a single-dose vial with a 30G needle and a filter needle. However, due to the dead space, much of the drug is not used is therefore wasted. The present invention provides an extraction method which produces multiple doses of ranibizumab from a single-dose vial. The doses produced are sterile, stable and effective for up to three months, less painful to the patient, and easier to use by a retinal specialist. The method described herein is not limited to ranibizumab and can be used for other drugs that have established stability under these condistions such as bevacizumab.
[0018]The following is a preferred embodiment for carrying out the method of the present invention.
[0019]Supply List (FIG. 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com