Methods of treating cancer using rad51 small molecule stimulators
a stimulator and small molecule technology, applied in the field of biochemistry, cell biology, oncology, can solve problems such as error-free repair, and achieve the effect of enhancing the toxic effect of imbalances in expression and activity levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of RAD51 Stimulators
[0113]Small molecule RAD51 stimulators were identified from a screen of a small-molecule chemical library as disclosed in Connell et. al. (US 2010 / 0248371), which is hereby incorporated by reference in its entirety. Briefly, a fluorescence polarization assay for RAD51 filament formation was used to screen a 10,000 compound small-molecule library (Chembridge DIVERSet collection) for compounds that stimulate RAD51 filament formation. The screen identified three small molecule compounds that stimulate RAD51 filament formation by at least 50% (FIGS. 8A-8E, compounds 45488 (“RS-1”), 43783, and 41936). Further study of RS-1 confirmed that it enhances RAD51 filament formation and that it protects these filaments from buffers containing high salt concentration (which typically destabilize RAD51 filaments). Imaging with electron microscopy confirmed that the increases in measured fluorescence polarization were, in fact, due to compound-stimulated filaments ...
example 2
Chemical Synthesis of Compounds
[0116]3-Benzylsulfamoyl-4-bromo-N-(4-bromo-phenyl)-benzamide was synthetized by reaction of chlorosulfonic acid with 4-bromobenzoic acid followed by sulfonamide formation with benzylamine and coupling with 4-bromoaniline. 1H NMR and 13C NMR spectra were obtained using a Bruker spectrometer with TMS as an internal standard. The following abbreviations indicating multiplicity were used: s=singlet, d=doublet, t=triplet, m=multiplet. HRMS experiments were carried out using a Shimadzu IT-TOF instrument with MeCN and H2O spiked with 0.1% formic acid as the mobile phase. Reaction progress was monitored by TLC using precoated silica gel plates (Merck silica gel 60 F254, 250 μm thickness). Preparative HPLC was carried out using a Shimadzu preparative liquid chromatograph with the following specifications: column, ACE 5 AQ (150 mm×21.2 mm) with 5 μm particle size; gradient, 25-100% MeOH / H2O, 30 min; 100% MeOH, 5 min; 100-25% MeOH / H2O, 4 min; 25% MeOH / H2O, 1 min;...
example 3
Low Levels of RAD54B and RAD54L Expression Area Associated with Sensitivity to RS-1 in Immortalized Human Cells
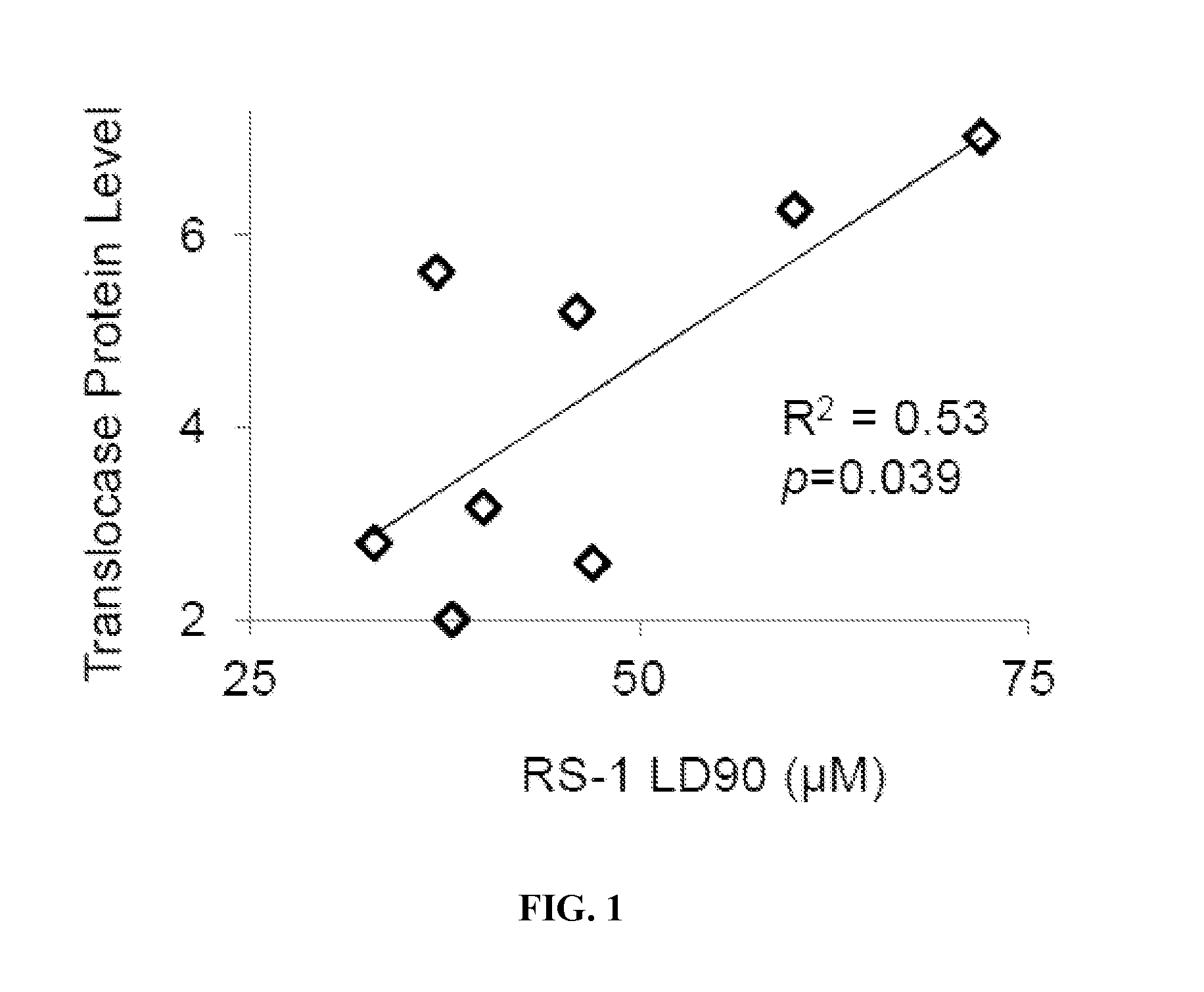
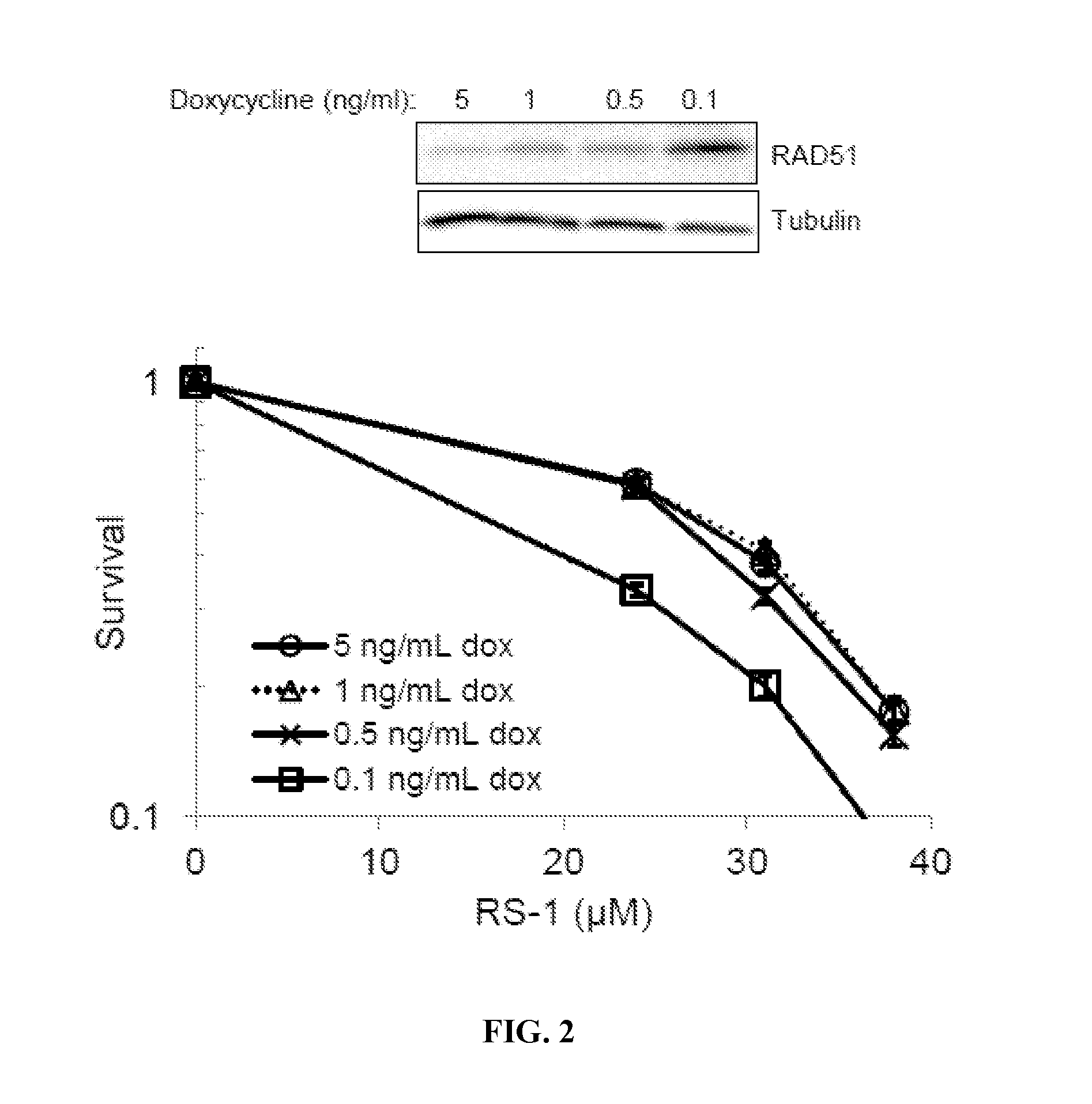
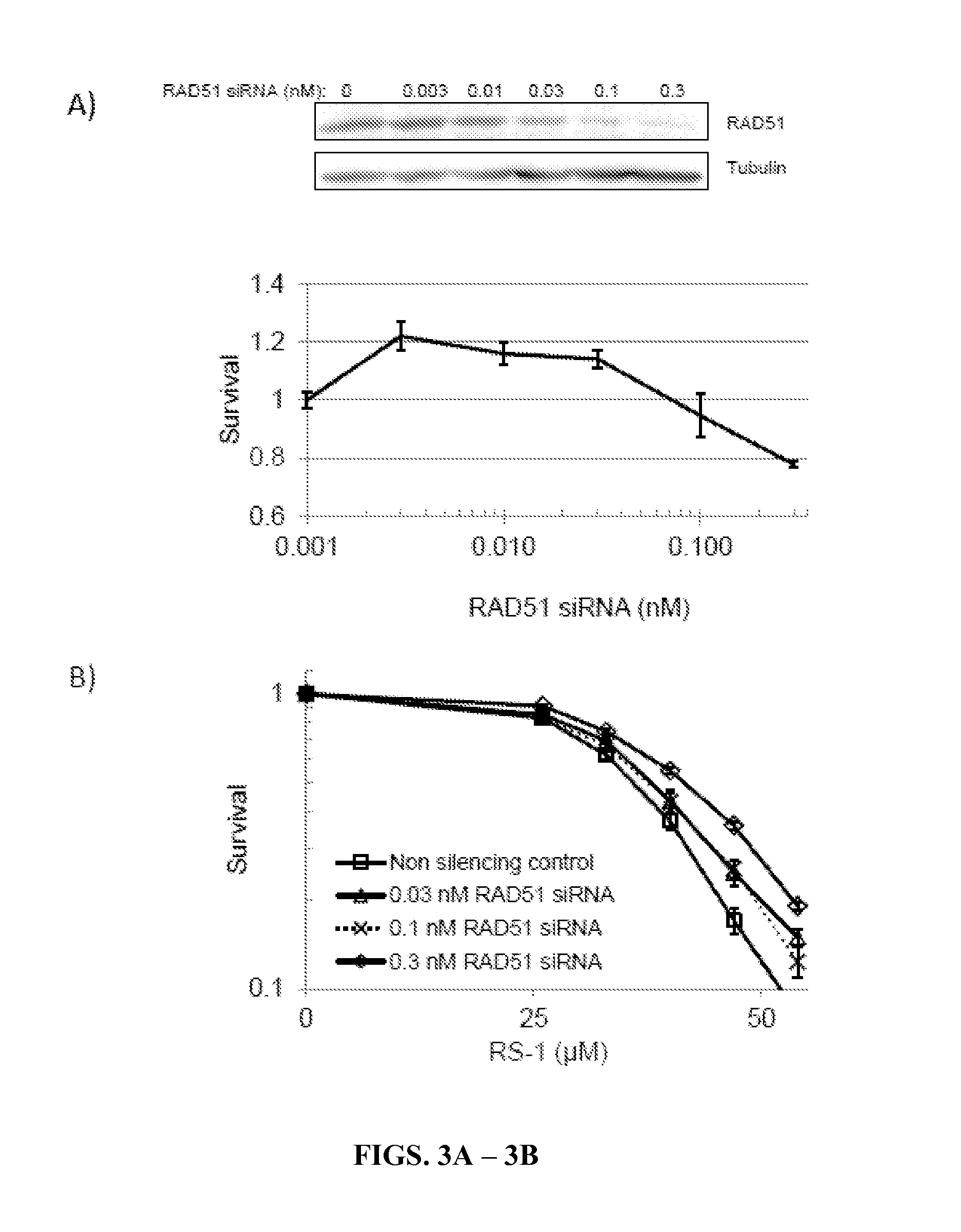
[0119]High levels of RAD51 overexpression render cells susceptible to the formation of toxic RAD51 complexes, particularly in cell types that harbor inadequate translocase activity (Shah, et al., 2010). The inventors examined whether malignant human cells with low / limiting levels of RAD54 translocase proteins would be hypersensitive to RS-1, a compound that increases the dNA binding activity of RAD51 (Jayathilaka, et al., 2008), using a panel of immortalized human cell lines. Whole cell levels for RAD51, RAD54L, and RAD54B proteins were measured by western blot, and the quantification for each cell line was normalized to levels in PC3 cells (Table 1). These relative protein levels were directly compared against RS-1 sensitivity (LD90 values) by linear regression analysis. The factor most strongly associated with RS-1 LD90 was RAD54B protein level (R2=0.33). By contrast, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com