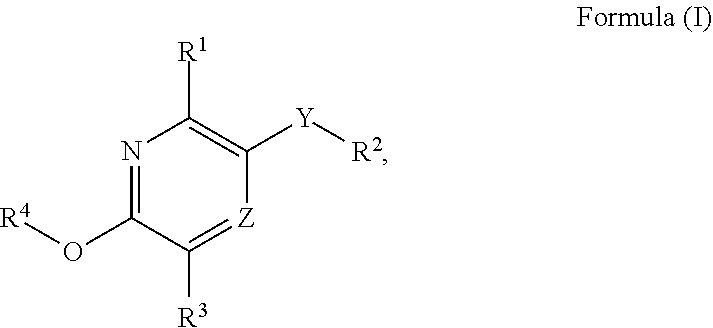
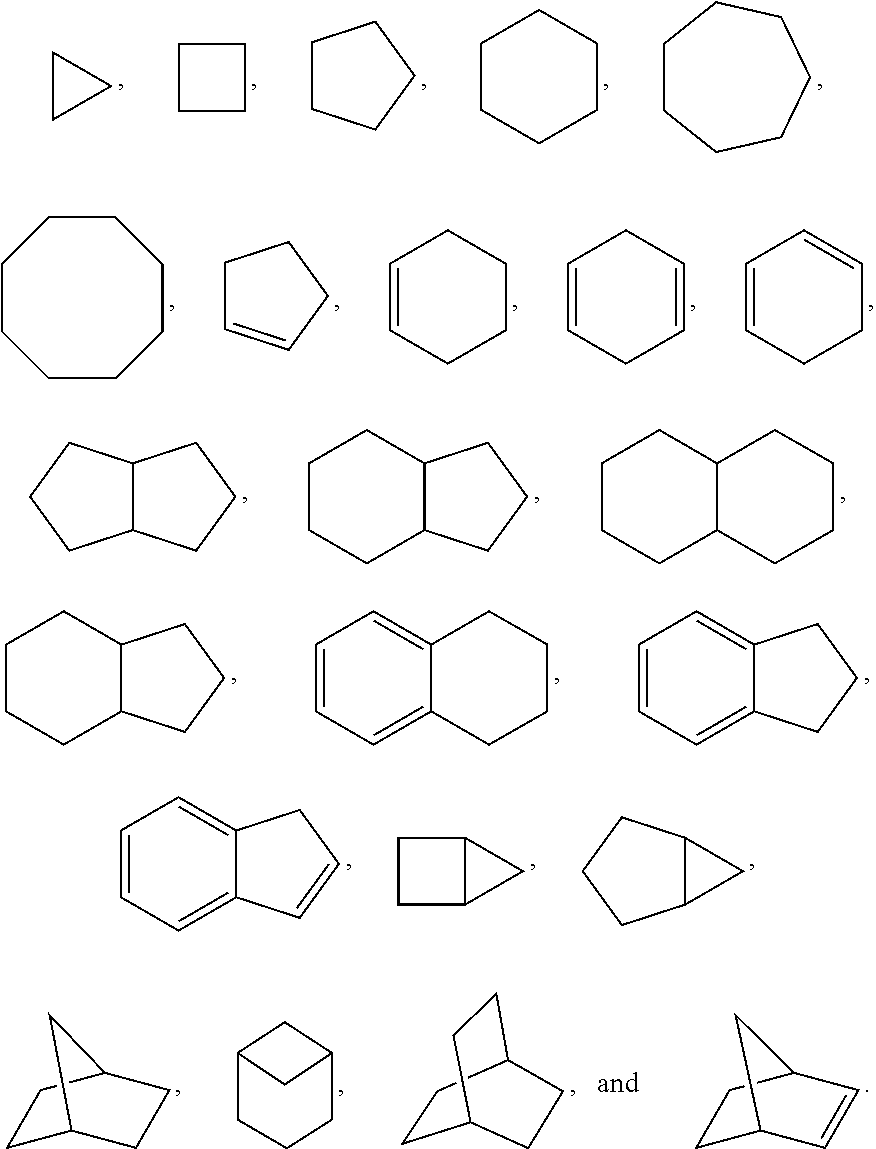
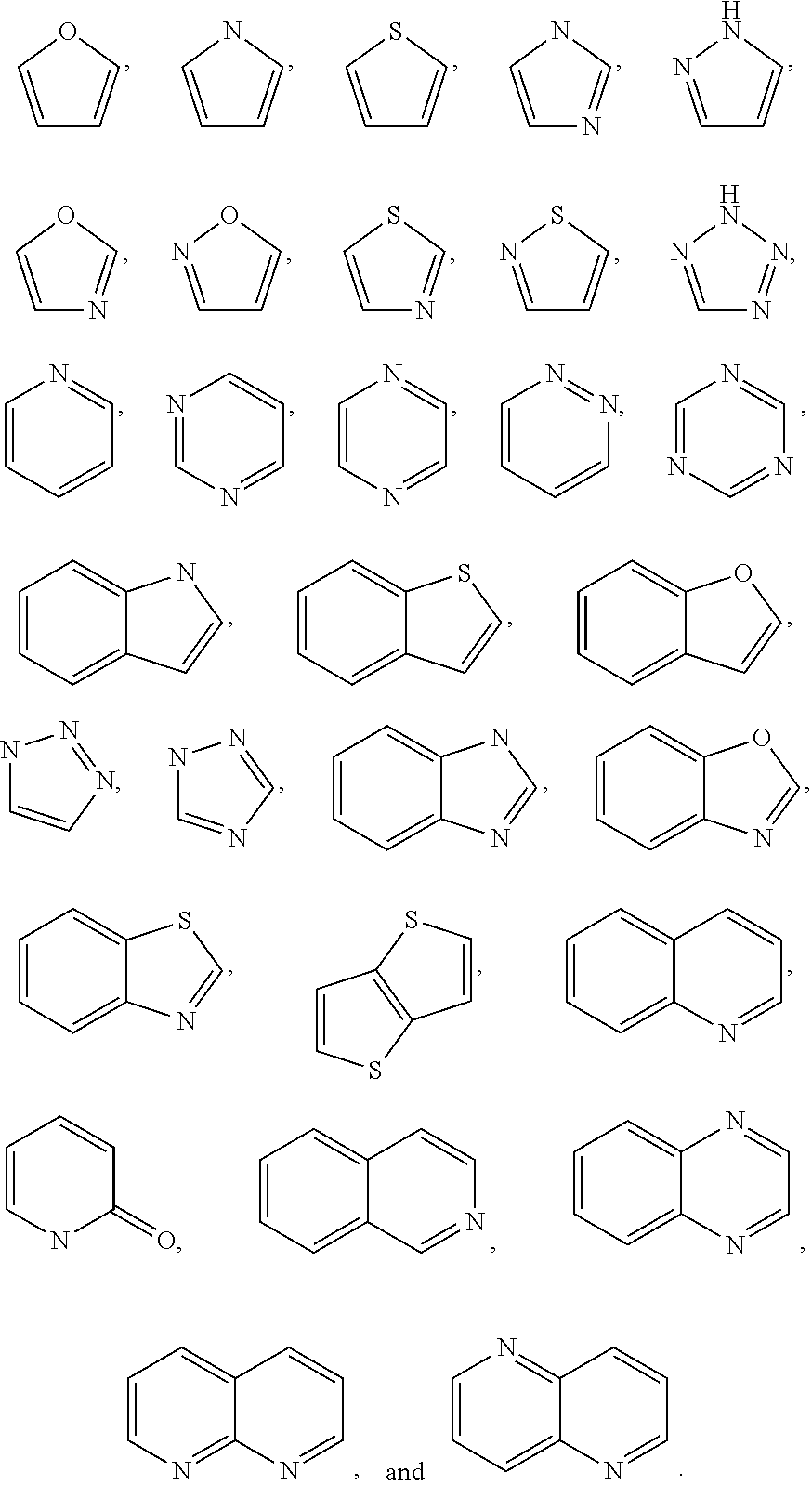
Substituted pyridine and pyrazine compounds as pde4 inhibitors
a technology of pyridine and pyrazine, which is applied in the direction of drug compositions, immune disorders, extracellular fluid disorders, etc., can solve the problems of general association of numerous side effects, etc., and achieves limited usefulness and tolerability, greater specificity, and higher potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
5-({6-[3-(Difluoromethoxy)phenyl]-5-ethoxypyrazin-2-yl}methyl)pyrimidine-2-carbonitrile
[0526]
[0527]Into a 5 mL microwave vial was combined 5-(bromomethyl)-3-(3-(difluoromethoxy)phenyl)-2-ethoxypyrazine (Intermediate 1, 176.00 mg, 0.49 mmol), 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine-2-carbonitrile (124.55 mg, 0.54 mmol), EtOH (2.45 mL), benzene (7.00 mL), Pd(PPh3)4 (56.63 mg, 0.05 mmol), and aq. NaHCO3 (1.38 mL, 1.15 mol / L, 1.59 mmol). The vial was sealed, purged with nitrogen and heated to 125° C. under microwave conditions for 15 minutes. Water was removed from the reaction with a pipette, and the crude reaction mixture was filtered thru CELITE®, and washed with EtOAc (3×5 mL). The combined organic layers were dried (Na2SO4), and the solvent was removed under reduced pressure. Purification (FCC, SiO2, 0-30%, EtOAc / hexanes) afforded the title compound as a white solid (100 mg, 53%). 1H NMR (400 MHz, CD3OD) δ 8.94 (s, 2H), 8.17 (s, 1H), 7.95-7.90 (m, 1H), 7.85 (t, J=...
example 2
2-Chloro-5-{[5-(3-chlorophenyl)-6-methoxypyridin-3-yl]methyl}pyrimidine
[0528]
[0529]To a solution of 5-(bromomethyl)-3-(3-chlorophenyl)-2-methoxypyridine (Intermediate 2, 100 mg, 0.321 mmol), (2-chloropyrimidin-5-yl)boronic acid (76 mg, 0.481 mmol) in ACN (3.2 mL) was added NaHCO3 (417 mg, 1.282 mmol) and PdCl2(dppf)-DCM (23 mg, 0.032 mmol). The reaction was heated under microwave conditions, at 120° C. for 12 minutes. Water was removed from the reaction with a pipette, and the crude reaction mix was filtered thru CELITE®, and washed with EtOAc. The combined organics were dried (Na2SO4), filtered and concentrated onto silica. Purification (FCC, SiO2, 30-70% EtOAc / hexanes) afforded the title compound (61 mg, 55%). 1H NMR (400 MHz, DMSO-d6) δ 8.34 (s, 2H), 8.11 (d, J=2.0 Hz, 1H), 7.70 (d, J=2.0 Hz, 1H), 7.58 (t, J=1.8 Hz, 1H), 7.53-7.36 (m, 3H), 3.83 (s 2H), 3.73 (s, 3H). [M+H]=346.11.
example 3
{2-[(5-{[5-(3-Chlorophenyl)-6-methoxypyridin-3-yl]methyl}pyrimidin-2-yl)amino]ethyl}dimethylamine
[0530]
[0531]To a solution of 2-chloro-5-{[5-(3-chlorophenyl)-6-methoxypyridin-3-yl]methyl}pyrimidine (Example 2, 50.00 mg, 0.14 mmol) in ACN (1.44 mL), was added N1,N1-dimethylethane-1,2-diamine (0.03 mL, 0.29 mmol), and DIPEA (77.13 μL, 0.43 mmol). The reaction mixture was heated at 180° C. for 15 minutes. EtOAc (5 mL) was added to the reaction mixture, and the reaction mixture was extracted with water (3×). The combined organic layers were dried (Na2SO4), and the solvent was removed under reduced pressure. Purification (FCC, SiO2, 0-15% MeOH / DCM) afforded the title compound (15.6 mg, 28%). 1H NMR (400 MHz, DMSO-d6) δ 8.21 (s, 2H), 8.09 (s, 1H), 7.67 (br s, 2H), 7.58 (s, 1H), 7.51-7.34 (m, 2H), 6.79 (br s, 1H), 3.84 (s, 3H), 3.72 (s, 2H), 2.42-2.37 (m, 4H), 2.17 (s, 6H). [M+H]=398.20.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Disorder | aaaaa | aaaaa |
| Enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com