Use of a dpp-4 inhibitor in sirs and/or sepsis
a dpp4 inhibitor and sepsis technology, applied in the field of dpp4 inhibitors, can solve the problems of microcirculatory dysfunction, abnormal coagulation and blood flow, excessive inflammation or immunosuppression, etc., and achieve the effect of reducing the risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
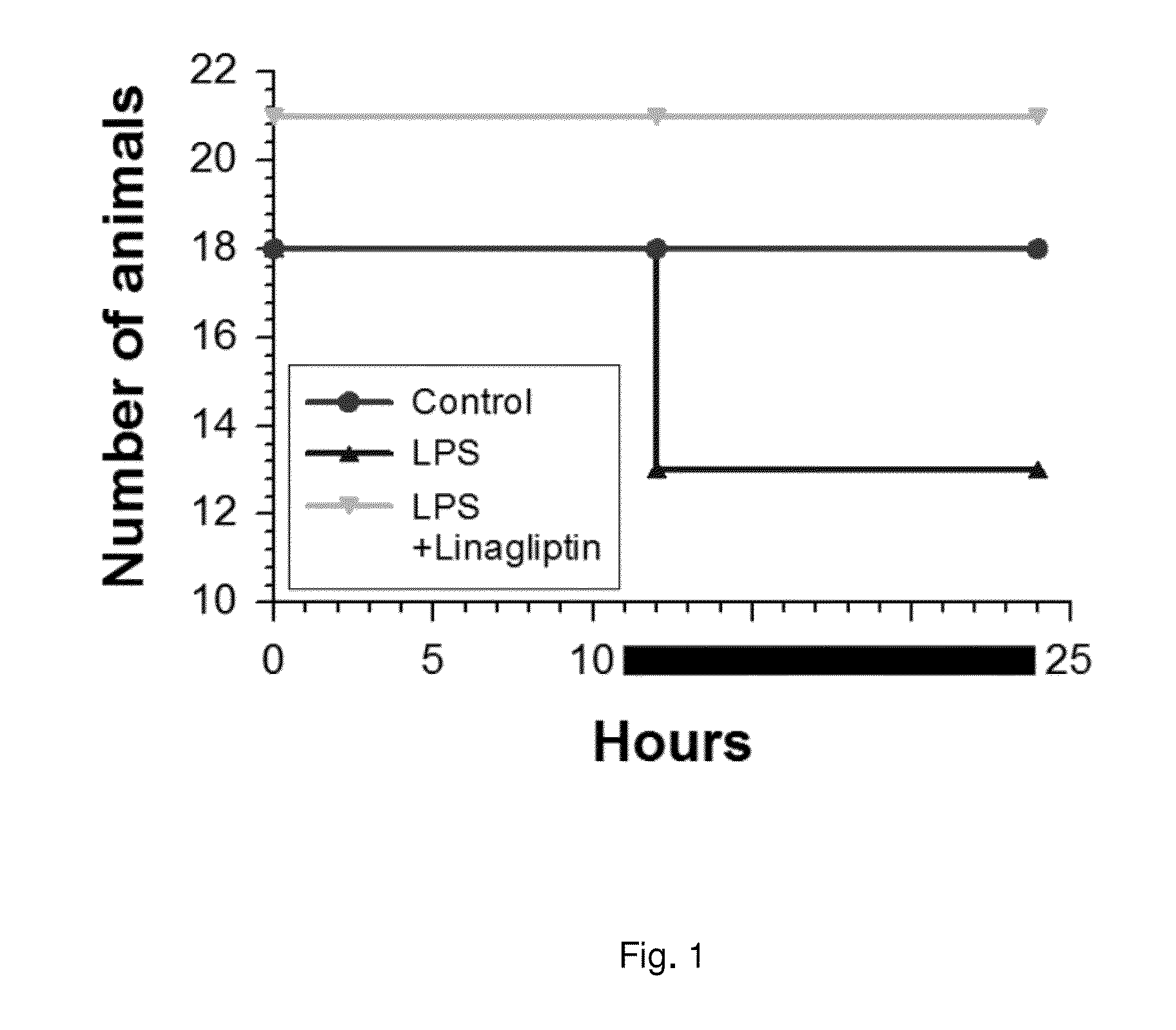
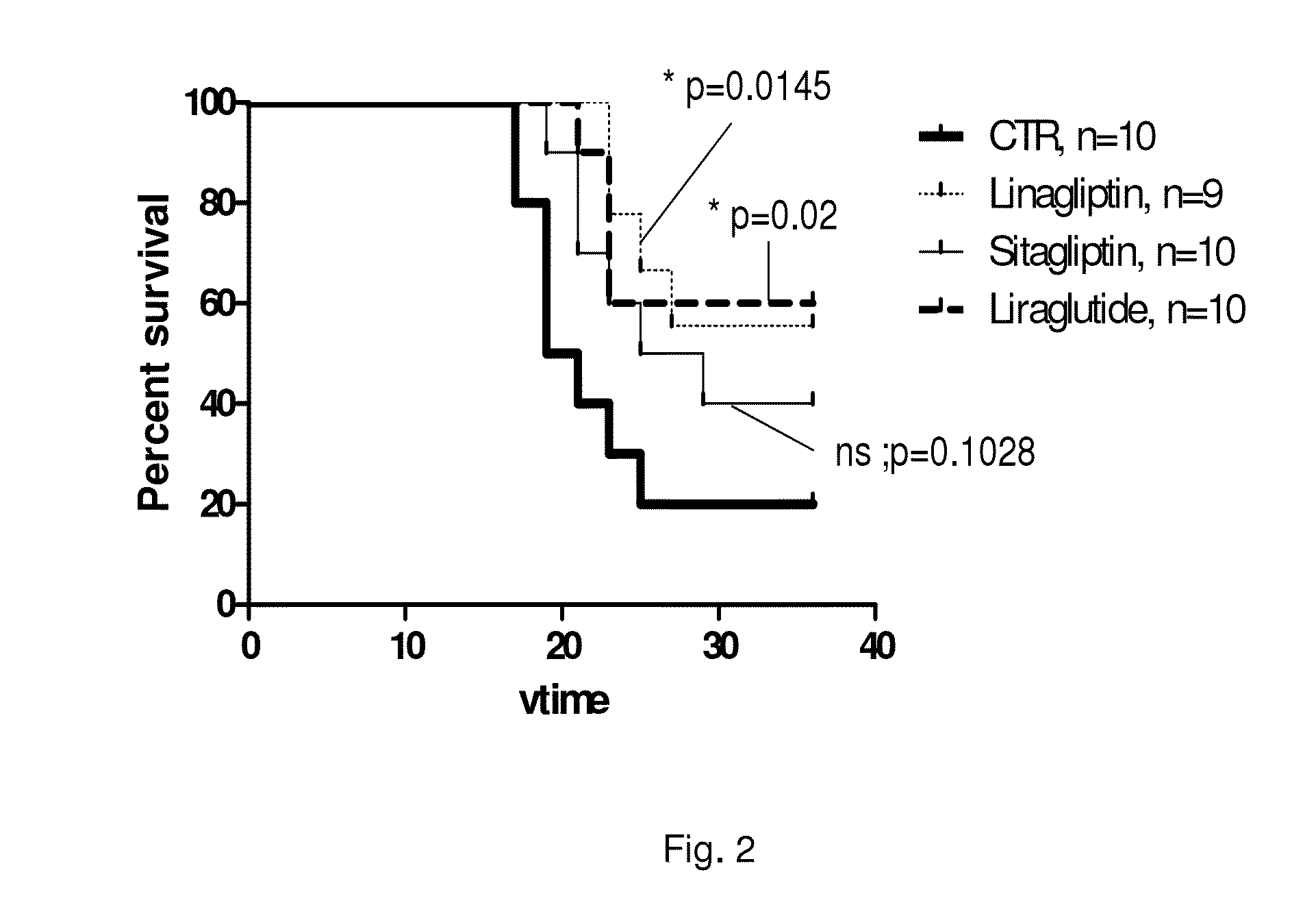
Survival of Septic Rats with and without Linagliptin Treatment
[0281]Female Wistar rats are fed a diet containing 0.083 mg / kg linagliptin (corresponding to 50-150 nM mean plasma concentration) for 6 day or control diet. Animals are injected at day 2 with 10 mg / kg LPS (lipopolysaccharide) i.p. and observed for 4 additional days.
[0282]Three groups of female Wistar rats are compared with respect to proportion surviving 24 hours LPS (lipopolysaccharide, 10 mg / kg i.p.) treatment using Fisher's exact test for 2 by 3 tables (p=0.004), and additionally, group b and c are compared using Fisher's two-sided exact test for 2 by 2 tables (p=0.015, LPS vs. LPS+Linagliptin). It should be noted that the survival curve only represents preliminary data (“pseudo Kaplan-Meier-curve”) since the exact time point of death of the rats is not assessed. The female Wistar rats are injected with LPS in the morning, monitored during the day and transferred back to the stable in the evening. Deaths are registered...
PUM
| Property | Measurement | Unit |
|---|---|---|
| waist circumference | aaaaa | aaaaa |
| waist circumference | aaaaa | aaaaa |
| waist circumference | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com