Self-Buffering Protein Formulations
a technology of protein formulation and buffer, which is applied in the field of self-buffering biopharmaceutical protein composition, can solve the problems of difficult to find a buffer, and the difficulty of finding a suitable buffer for pharmaceutical use can be especially acu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
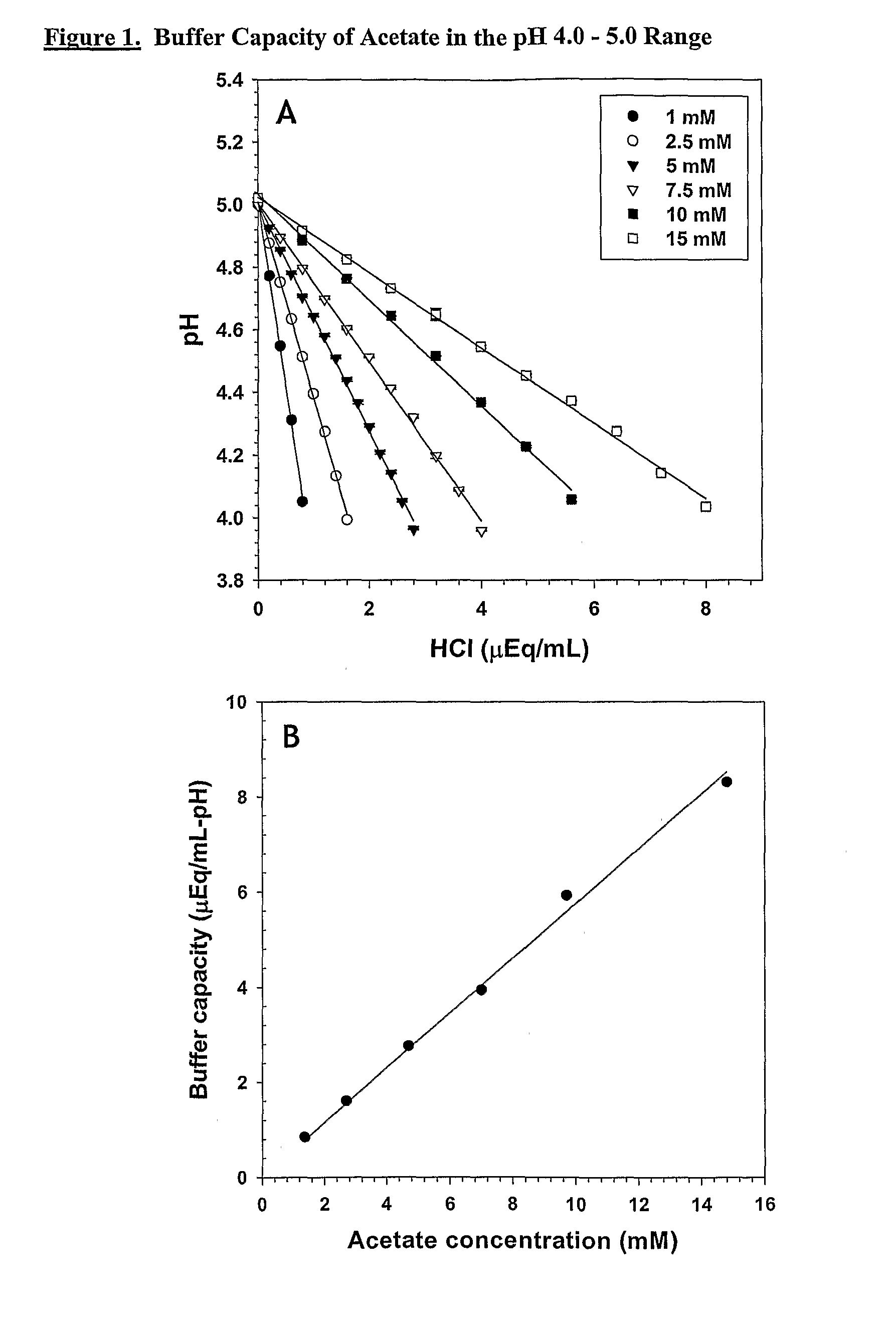
Acid Titrations and Buffer Capacities of Sodium Acetate Buffers in the Range pH 5.0 to 4.0
[0375]A stock solution of known concentration of acetic acid was prepared by diluting ultrapure glacial acetic acid in HPLC grade water and then titrating the pH up to the desired value with NaOH. Stocks were equilibrated to the air and to 21° C. Volumetric standards were prepared at a concentration of 1 N and diluted as necessary with HPLC water.
[0376]One mM, 2.5 mM, 5 mM, 7.5 mM, 10 mM, and 15 mM sodium acetate buffers were prepared by diluting the stock in HPLC water. The solutions were titrated with HCl. 0.2 N HCl was used for the 1, 2.5, and 5 mM solutions, 0.4 N HCl was used for the 7.5 mM solution, and 0.8 N HCl was used for the 10 and 15 mM solutions. The titrations were performed using standard analytical laboratory techniques.
[0377]FIG. 1, Panel A shows the titration data and the least squares trend lines calculated from the data for each solution. The slope of the trend line calculat...
example 2
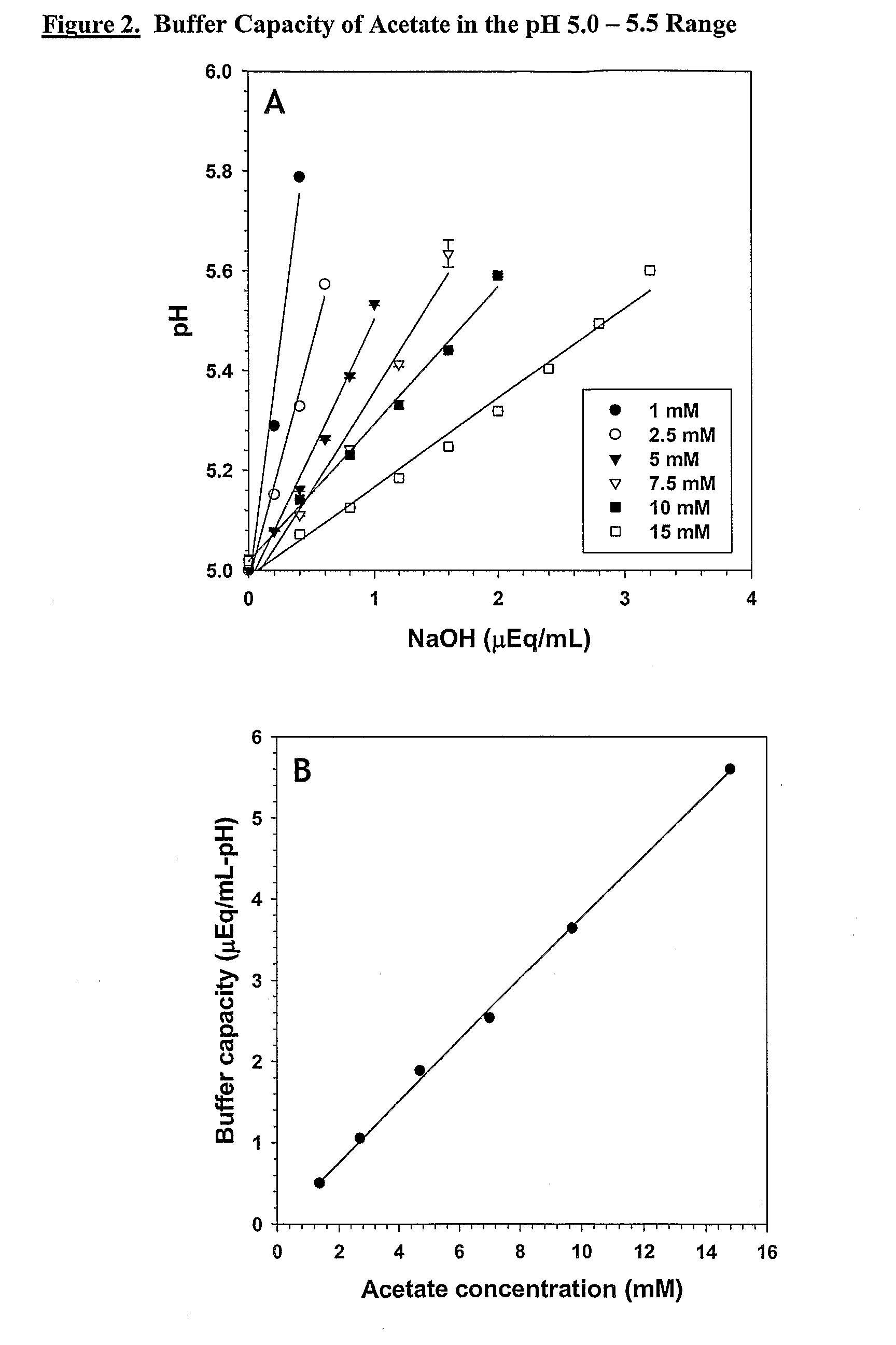
Base Titrations and Buffer Capacities of Sodium Acetate Buffers in the Range pH 5.0 to 5.5
[0378]Acetate buffer stocks and solutions for titration were prepared as described in Example 1. The solutions were titrated as described in Example 1, except that the solutions were titrated from pH 5.0 to 5.5 and the titrations were done using NaOH instead of HCl. 0.2 N NaOH was used to titrate the 1, 2.5, and 5 mM solutions and 0.4N NaOH was used for the 7.5, 10, and 15 mM solutions. The results of the titrations are shown in FIG. 2A. The linear dependence of buffer capacity on concentration of acetate buffer is displayed in FIG. 2B.
example 3
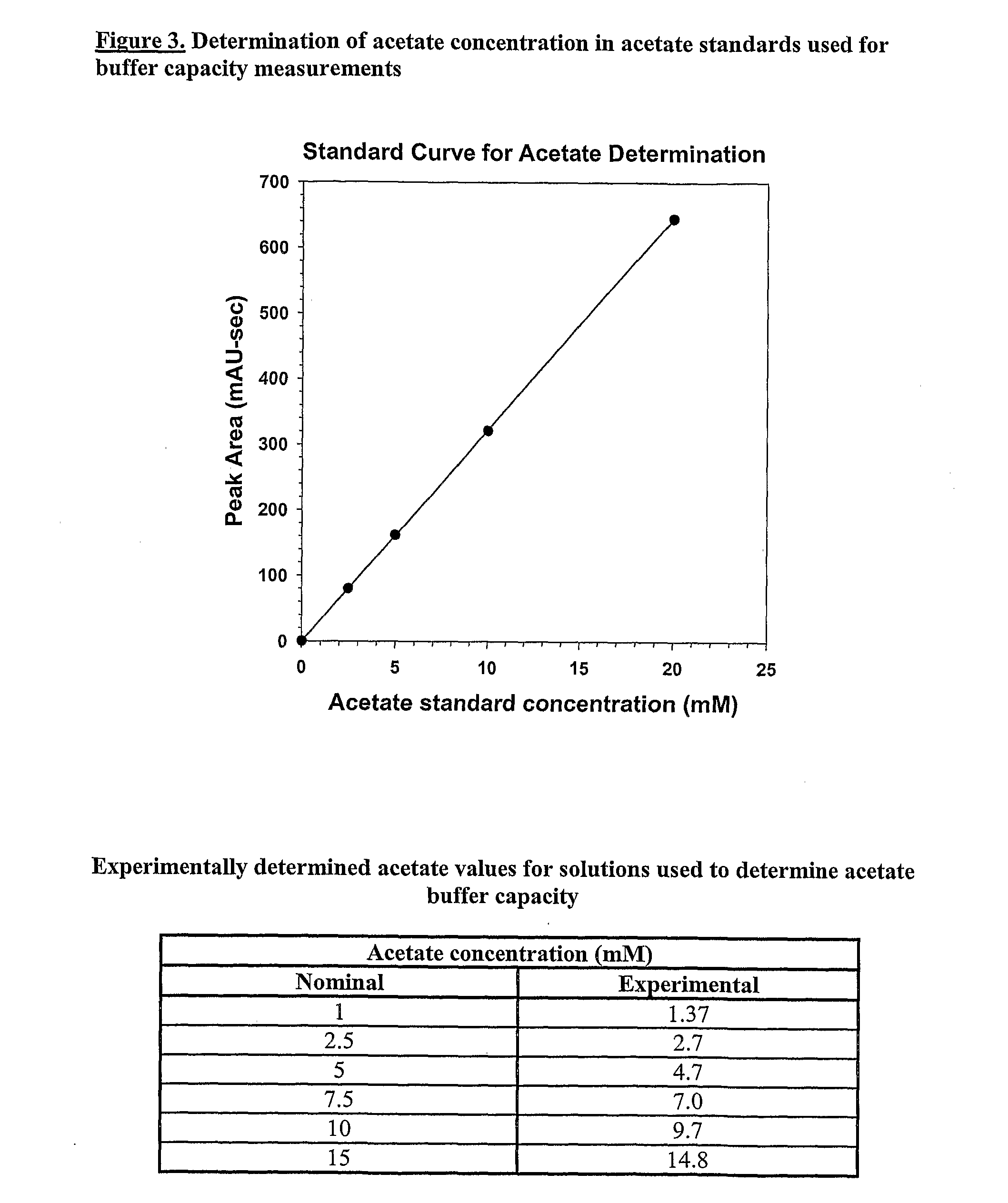
Determination of Acetate by HPLC
[0379]Acetate was determined in acetate buffer samples using analytical SE-HPLC. A standard curve for peak areas as a function of acetate concentration was established by analysis of acetate in buffers of known acetate concentration. The amount of acetate in test samples was interpolated from the standard curve. A standard curve is shown in FIG. 3. Nominal and measured amount of acetate in test buffers are tabulated below the standard curve in the figure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com