Implantable polymeric device for sustained release of dopamine agonist
a polymeric device and agonist technology, applied in the direction of prosthesis, drug composition, powder delivery, etc., can solve the problems of inconvenient administration, abnormalities, and technical difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Materials
[0047]The following materials were used:[0048]Apomorphine HCl, supplied by Hawkins, Inc.[0049]Triamcinolone Acetonide, supplied by Spectrum[0050]Glutathione, supplied by Aldrich, St. Louis, Mo.[0051]Ethylene vinyl acetate copolymer, 33% vinyl acetate, supplied by Southwest Research Institute, San Antonio, Tex.[0052]Methanol, ChromAR HPLC Grade, supplied by Mallinckrodt, St. Louis, Mo.[0053]Acetonitrile, ChromAR HPLC. Grade, supplied by Mallinckrodt, St. Louis, Mo.[0054]Trifluoro Acetic Acid, 99%, Spectrochemical grade, supplied by Aldrich Chemicals, St. Louis, Mo.[0055]Sodium Dodecyl Sulfate, 99%, supplied by EM Science Ethanol, supplied by Mallinckrodt, St. Louis, Mo.
HPLC Assays
[0056]An HPLC method was used to determine the rate of in vitro release of apomorphine HCl (“ApoH”) or loratidine (“LA”) from the implants. Chromatography was performed using a Zorbax SB-C18 (250 mm×4.6 mm) column and 60% 0.1 trifluoro acetic acid in water, 15% methanol, 25% ace...
example 2
In Vitro Characterization of Extruded Implantable Devices
[0060]Extruded rods prepared as described above were characterized for total drug load and for rate of drug release.
Assessment of Drug Loading
[0061]Implants prepared with 70% ApoH:30% EVA were cut into 2 mm pieces, accurately weighed, and placed into 250 ml volumetric flasks. Approximately 200 ml of methanol was added to each flask and the solution was continuously stirred at room temperature until the implants was dissolved. The solution was then assayed for drug content.
[0062]The average ApoH content for unwashed, washed, and sterilized rods was between 66.9 and 67.9% ApoH, corresponding to 95.6 to 97% recovery.
Assessment of Drug Release
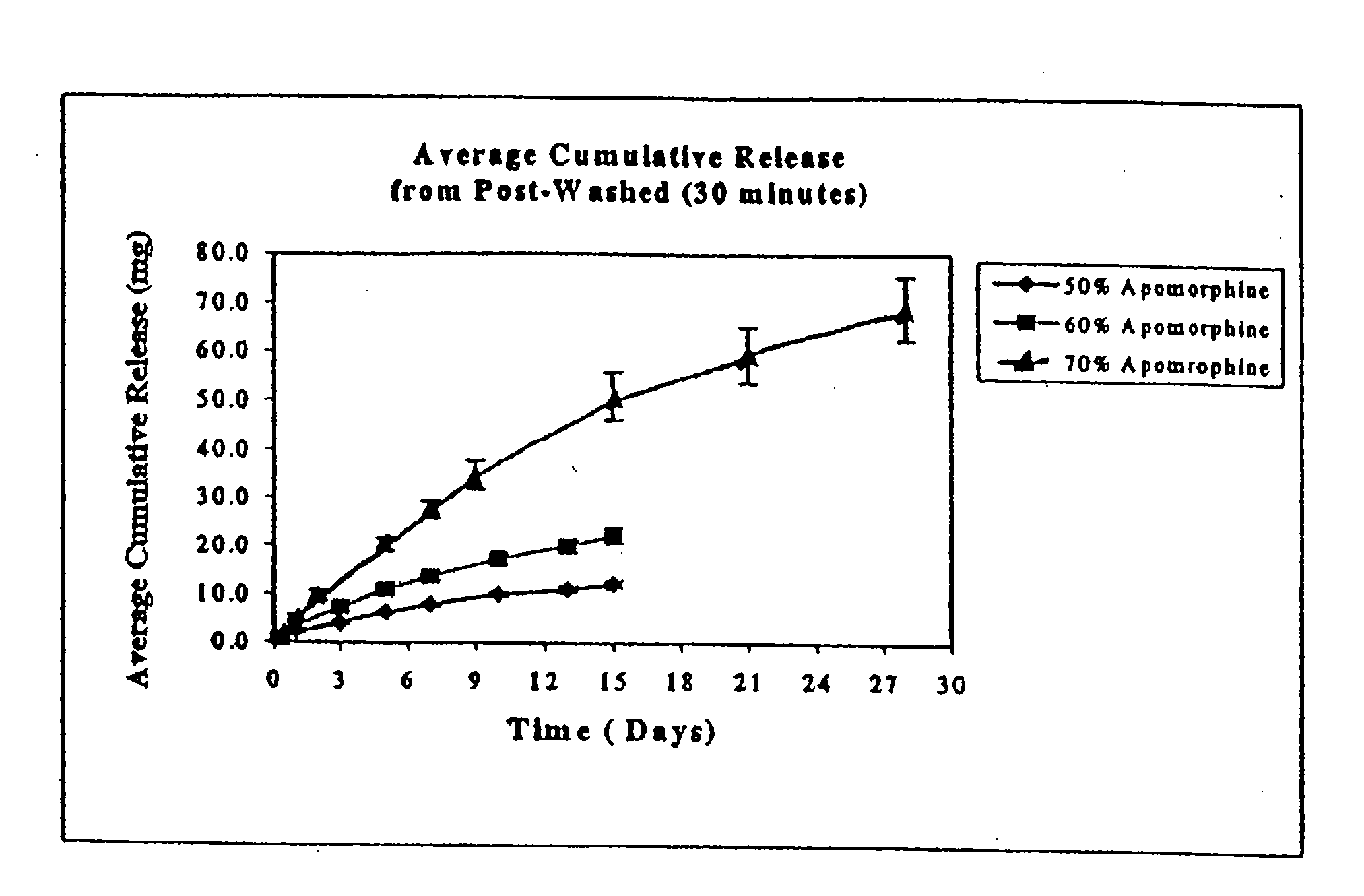
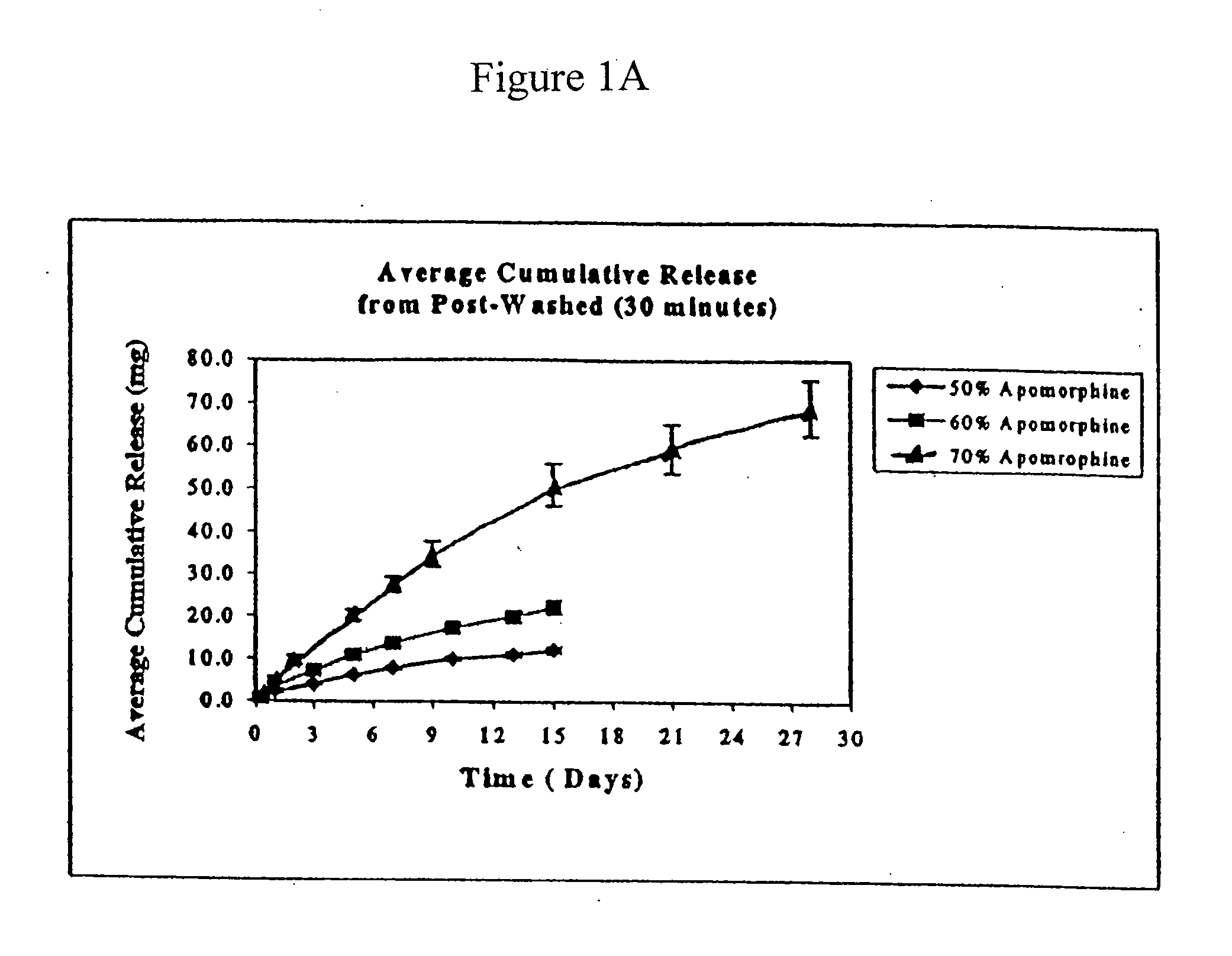
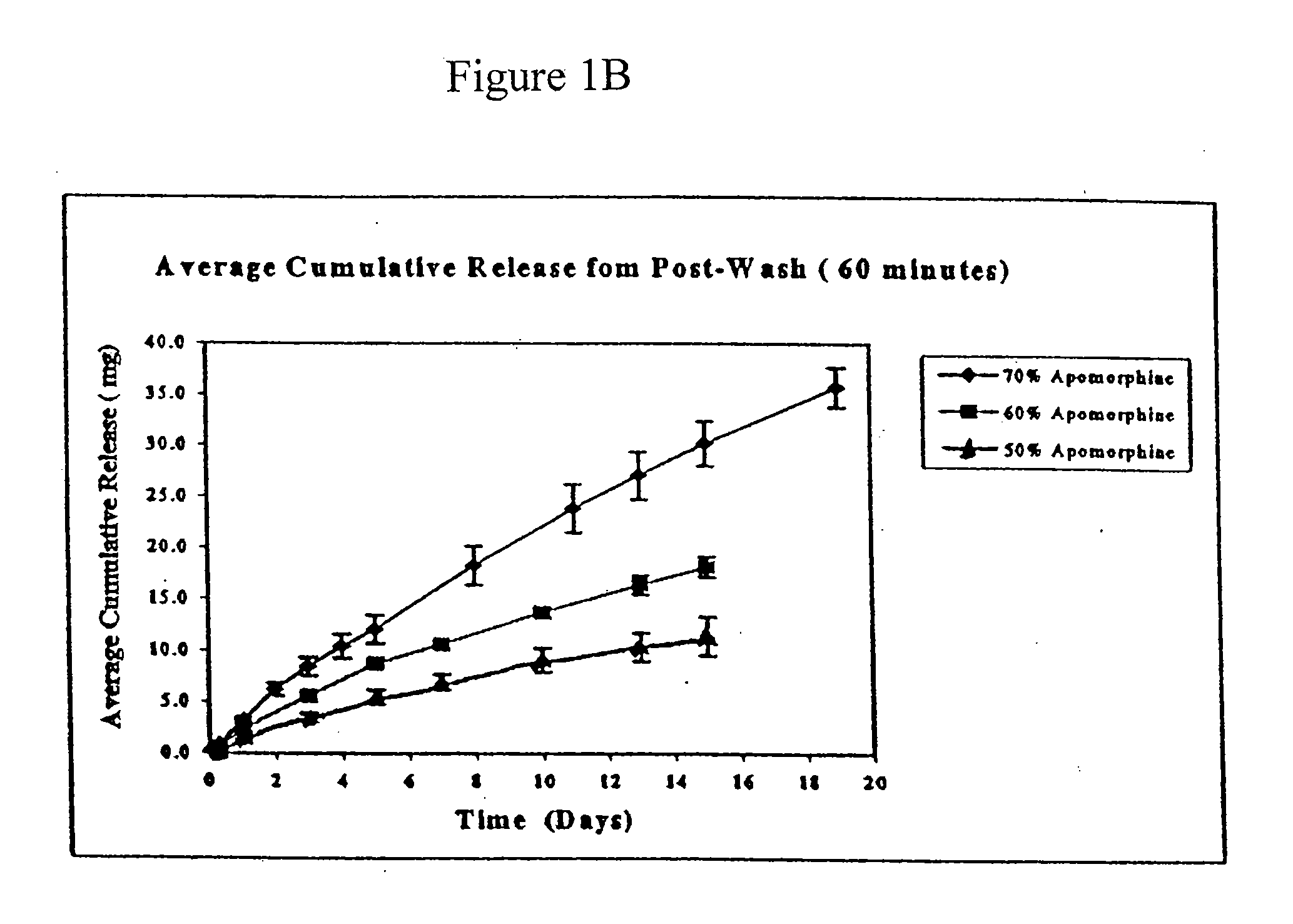
[0063]Experiments were performed to determine the rate of apomorphine released from the extruded rods. The medium for these studies was 0.5% sodium dodecyl sulfate (“SDS”). Preweighed rods were placed in 100 ml screw cap jars containing 50 ml of medium and placed on an orbital shaker. The orb...
example 3
In Vivo Evaluation of Drug Loaded Implantable Devices
[0066]Four MPTP-lesioned, L-DOPA-naïve cynomolgus monkeys were administered three 2.4 mm diameter×2.6 cm length rod-shaped implantable devices prepared as described above, each containing 33% vinyl acetate and loaded with 98 mg ±10% apomorphine HCl (68.5% apomorphine). Devices were implanted between the shoulder blades using a trocar. For comparison, three additional MPTP-lesioned, L-DOPA-naïve monkeys received pulsatile daily subcutaneous injections of apomorphine at a dosage of 0.2 mg / kg, which is the minimally-effective dose to achieve “ON” status in the animal.
[0067]All of the monkeys that received apomorphine implants were continuously in an “ON” state within one day after implantation, with an average steady state apomorphine level of approximately 0.5-1.0 ng / ml achieved after an initial burst. In contrast, animals that received pulsatile injections were “ON” for only approximately 90 minutes after each administration of apo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com