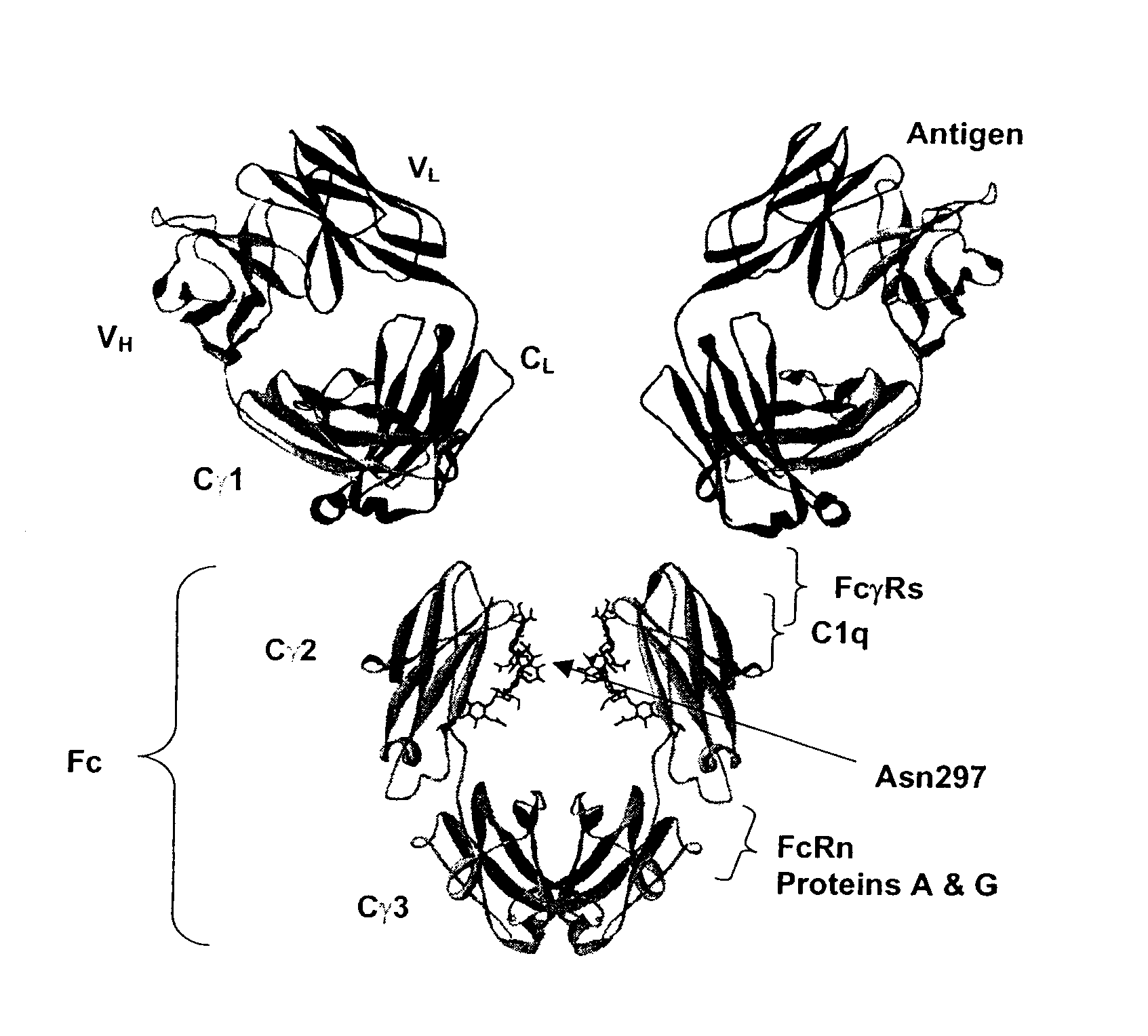
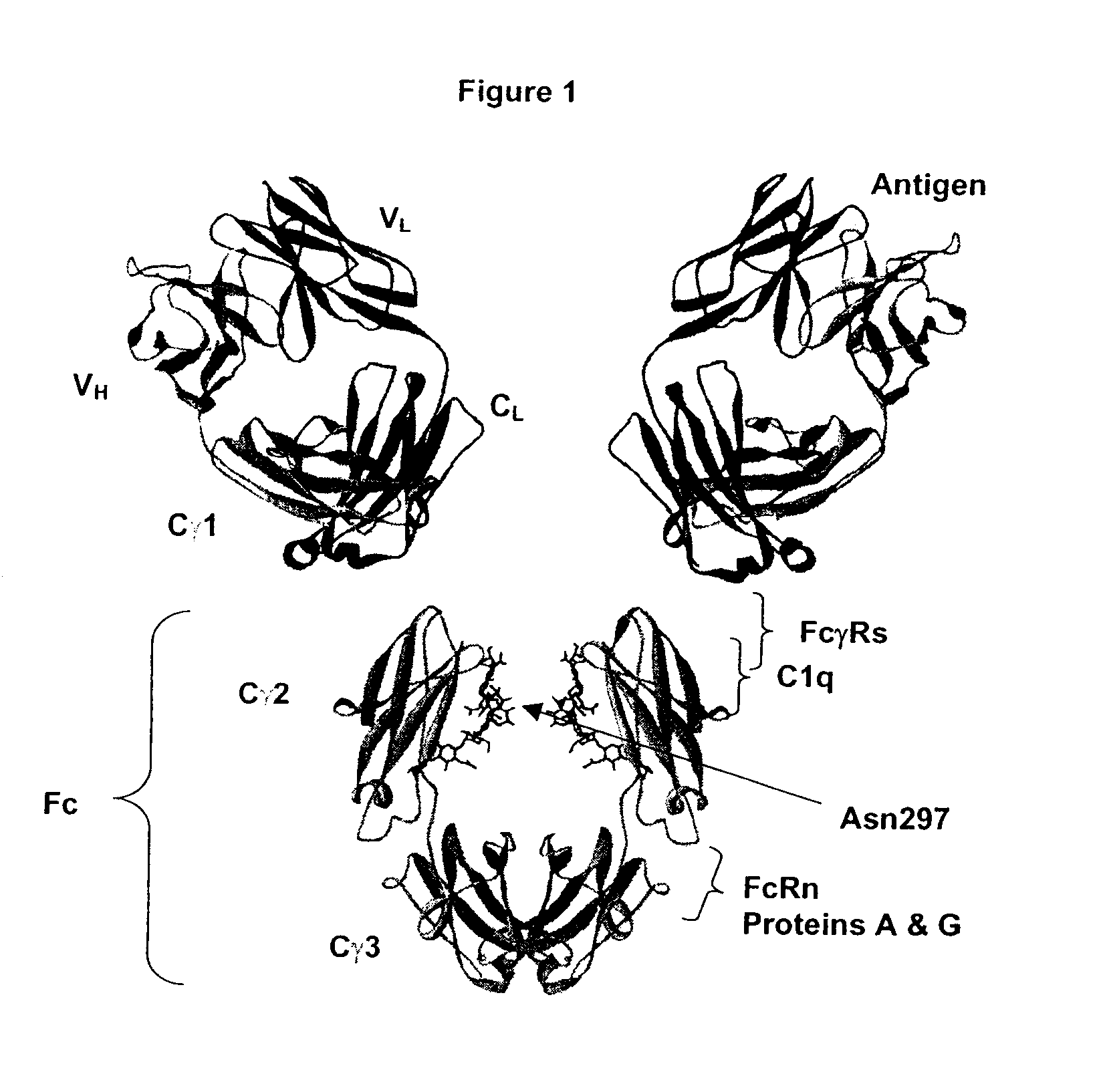
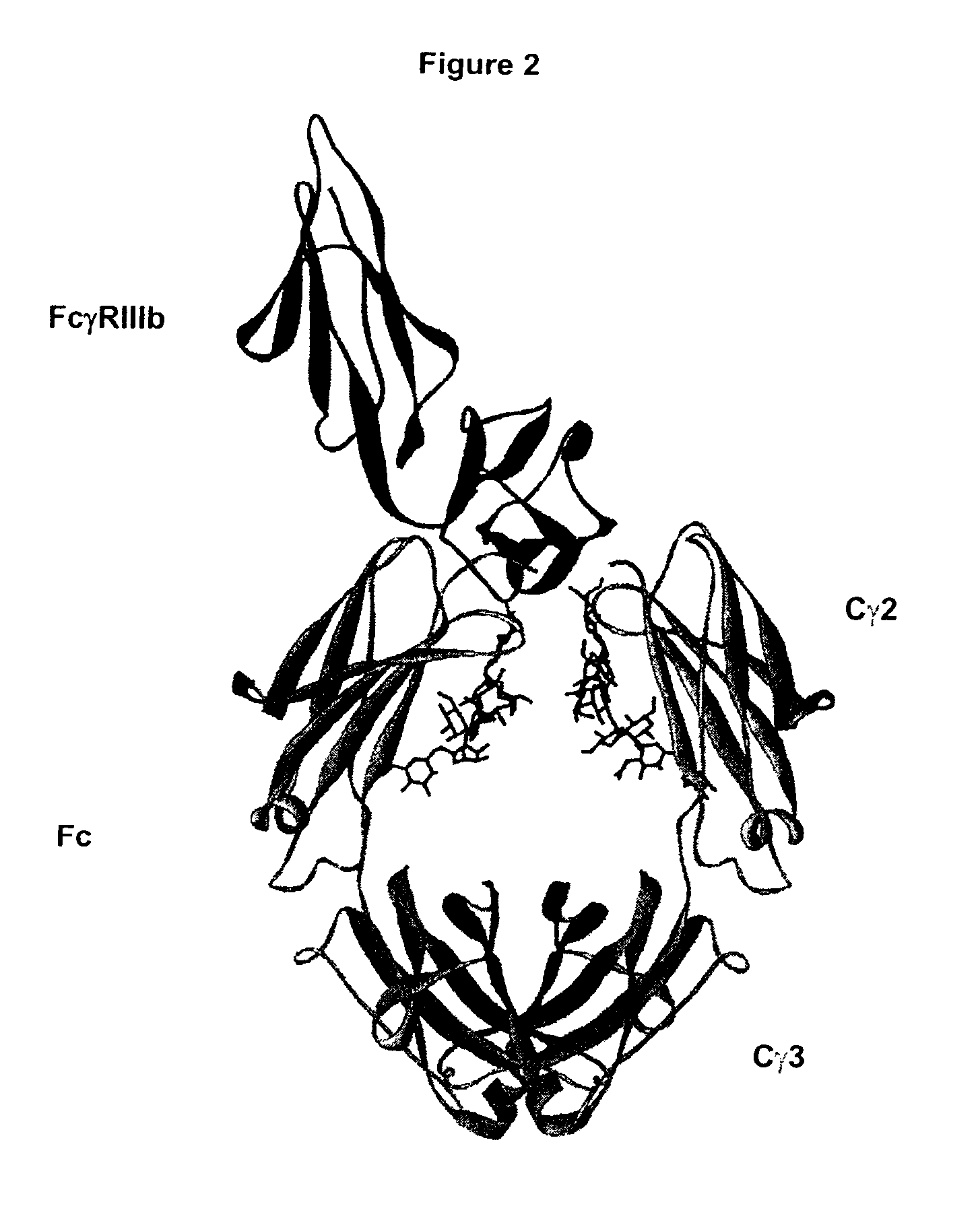
Fc Variants Having Decreased Affinity for FcyRlla
a variant and affinity technology, applied in the field of new optimized variants, can solve the problems of unsatisfactory potency of antibodies as anti-cancer agents, another level of complexity, and biotherapeutics consume essentially all of the available manufacturing capacity, and achieve the effects of reducing the affinity, improving the function and/or solution properties, and facilitating the effect of effector function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Molecular Biology and Protein Expression / Purification
[0250] The majority of experimentation on the Fc variants was carried out in the context of the anti-cancer antibody alemtuzumab (Campath®, a registered trademark of Ilex Pharmaceuticals LP). Alemtuzumab binds a short linear epitope within its target antigen CD52 (Hale et al., 1990, Tissue Antigens 35:118-127; Hale, 1995, Immunotechnology 1:175-187). Alemtuzumab has been chosen as the primary engineering template because its efficacy is due in part to its ability to recruit effector cells (Dyer et al., 1989, Blood 73:1431-1439; Friend et al., 1991, Transplant Proc 23:2253-2254; Hale et al., 1998, Blood 92:4581-4590; Glennie et al., 2000, Immunol Today 21:403-410), and because production and use of its antigen in binding assays are relatively straightforward. In order to evaluate the optimized Fc variants of the present invention in the context of other antibodies, select Fc variants were evaluated in the anti-Her2 antibody trastu...
example 2
Fc Ligand Binding Assays
[0254] Binding to the human Fc ligands FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa, C1q, and FcRn was measured for the designed Fc variants. Binding affinities were measured using an AlphaScreen™ assay (Amplified Luminescent Proximity Homogeneous Assay (ALPHA), PerkinElmer, Wellesley, Mass.), a bead-based luminescent proximity assay. Laser excitation of a donor bead excites oxygen, which if sufficiently close to the acceptor bead generates a cascade of chemiluminescent events, ultimately leading to fluorescence emission at 520-620 nm. WT alemtuzumab antibody was biotinylated by standard methods for attachment to streptavidin donor beads, and GST-tagged FcγRs and FcRn were bound to glutathione chelate acceptor beads. For the C1q binding assay, untagged C1q protein was conjugated with Digoxygenin (DIG, Roche) using N-hydrosuccinimide (NHS) chemistry and bound to DIG acceptor beads. For the protein A binding assay, protein A acceptor beads were purchased directl...
example 3
ADCC of Fc Variants
[0267] In order to determine the effect on effector function, cell-based ADCC assays were performed on select Fc variants. ADCC was measured using the DELFIA® EuTDA-based cytotoxicity assay (Perkin Elmer, Mass.) with purified human peripheral blood monocytes (PBMCs) as effector cells. Target cells were loaded with BATDA at 1×106 cells / ml, washed 4 times and seeded into 96-well plate at 10,000 cells / well. The target cells were then opsonized using Fc variant or WT antibodies at the indicated final concentration. Human PBMCs, isolated from buffy-coat were added at the indicated fold-excess of target cells and the plate was incubated at 37° C. for 4 hrs. The co-cultured cells were centrifuged at 500×g, supernatants were transferred to a separate plate and incubated with Eu solution, and relative fluorescence units were measured using a Packard Fusion™α-FP HT reader (Packard Biosciences, Ill.). Samples were run in triplicate to provide error estimates (n=3, + / −S.D.)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com