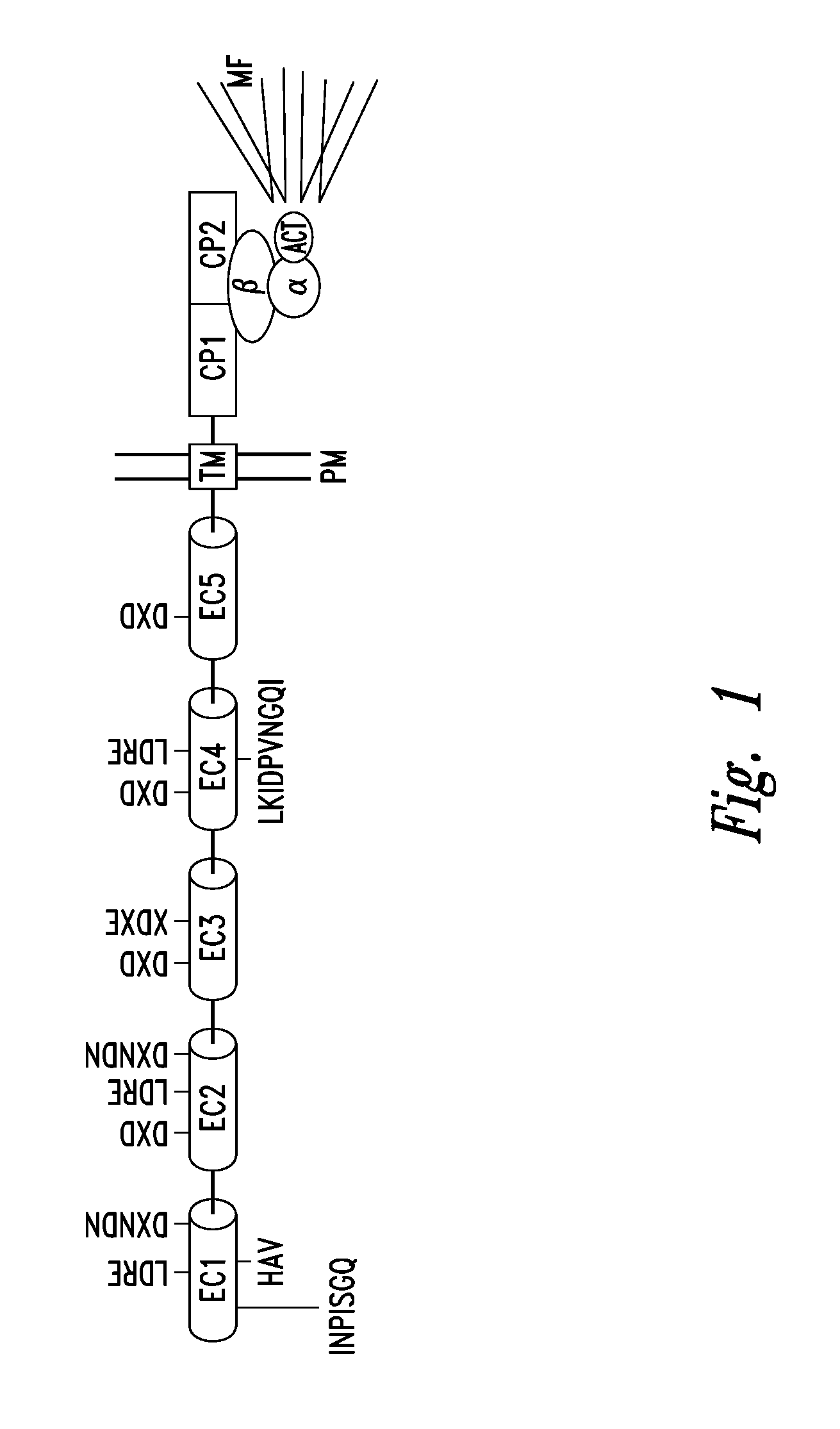
Compounds and methods for modulating adhesion molecule function
a cadherin-mediated process and compound technology, applied in the direction of antibody medical ingredients, peptide/protein ingredients, drug compositions, etc., can solve the problems of limited use of skin patches, hydrophobic molecules, and undesirable cell adhesion levels, so as to prevent pregnancy, inhibit synaptic stability, and increase vasopermeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Representative Modulating Agents
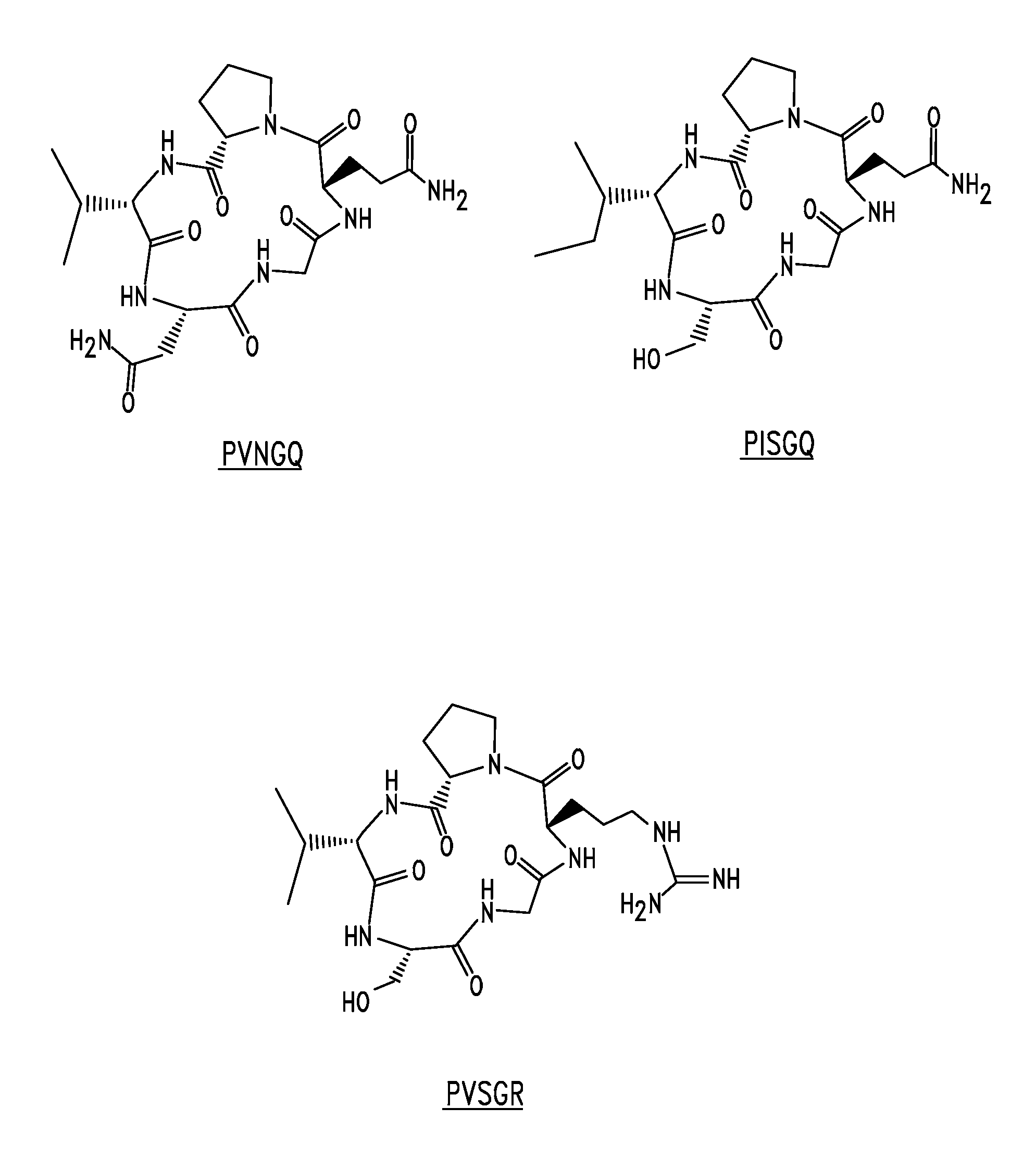
[0202] This Example illustrates the solid phase synthesis of representative peptide modulating agents.
[0203] The peptides were synthesized on a 431A Applied Biosystems peptide synthesizer using p-Hydroxymethylphenoxymethyl polystyrene (HMP) resin and standard Fmoc chemistry. After synthesis and deprotection, the peptides were de-salted on a Sephadex G-10 column and lyophilized. The peptides were analyzed for purity by analytical HPLC, and in each case a single peak was observed. Peptides were made as stock solutions at 10 to 25 mg / mL in dimethylsulfoxide (DMSO) or water and stored at −20° C. before use.
example 2
Disruption of the Ability of Mouse Cerebellar Neurons to Extend Neurites
[0204] N-cadherin and N-CAM are established as CAMs that can regulate neurite outgrowth (Doherty and Walsh, Curr. Op. Neurobiol. 4:49-55, 1994; Williams et al., Neuron 13:583-594, 1994; Hall et al., Cell Adhesion and Commun. 3:441-450, 1996; Doherty and Walsh, Mol. Cell. Neurosci. 8:99-111, 1996; Saffell et al., Neuron 18:231-242, 1997). Neurons cultured on monolayers of 3T3 cells that have been transfected with cDNAs encoding N-cadherin or N-CAM extend longer neurites than neurons cultured on the untransfected parental 3T3 cells (commonly referred to as the control 3T3 cells). It has been determined that the neurite response stimulated by transfected N-CAM and N-cadherin initially depends upon a trans homophilic binding interaction between the transfected CAM in the 3T3 cell and the corresponding CAM in the neuron. This Example illustrates the use of representative modulating agents to disrupt neurite outgrowt...
example 3
Modulating Agent Binding to N-Cadherin
[0213] This Example illustrates the ability of a representative modulating agent to bind to N-cadherin.
[0214] The peptide H-WLKIDPVNGQI-OH (SEQ ID NO:13) was passed over flow cells coated with an N-cadherin-Fc chimera or human IgG1 at a concentration of either 250, 500 or 1000 μg / ml. FIG. 7 is a graph illustrating the association of the peptide to the flow cell coated with the N-cadherin Fc chimera, with the binding to the control flow cell (coated with human IgG1) automatically subtracted.
PUM
| Property | Measurement | Unit |
|---|---|---|
| RG | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com