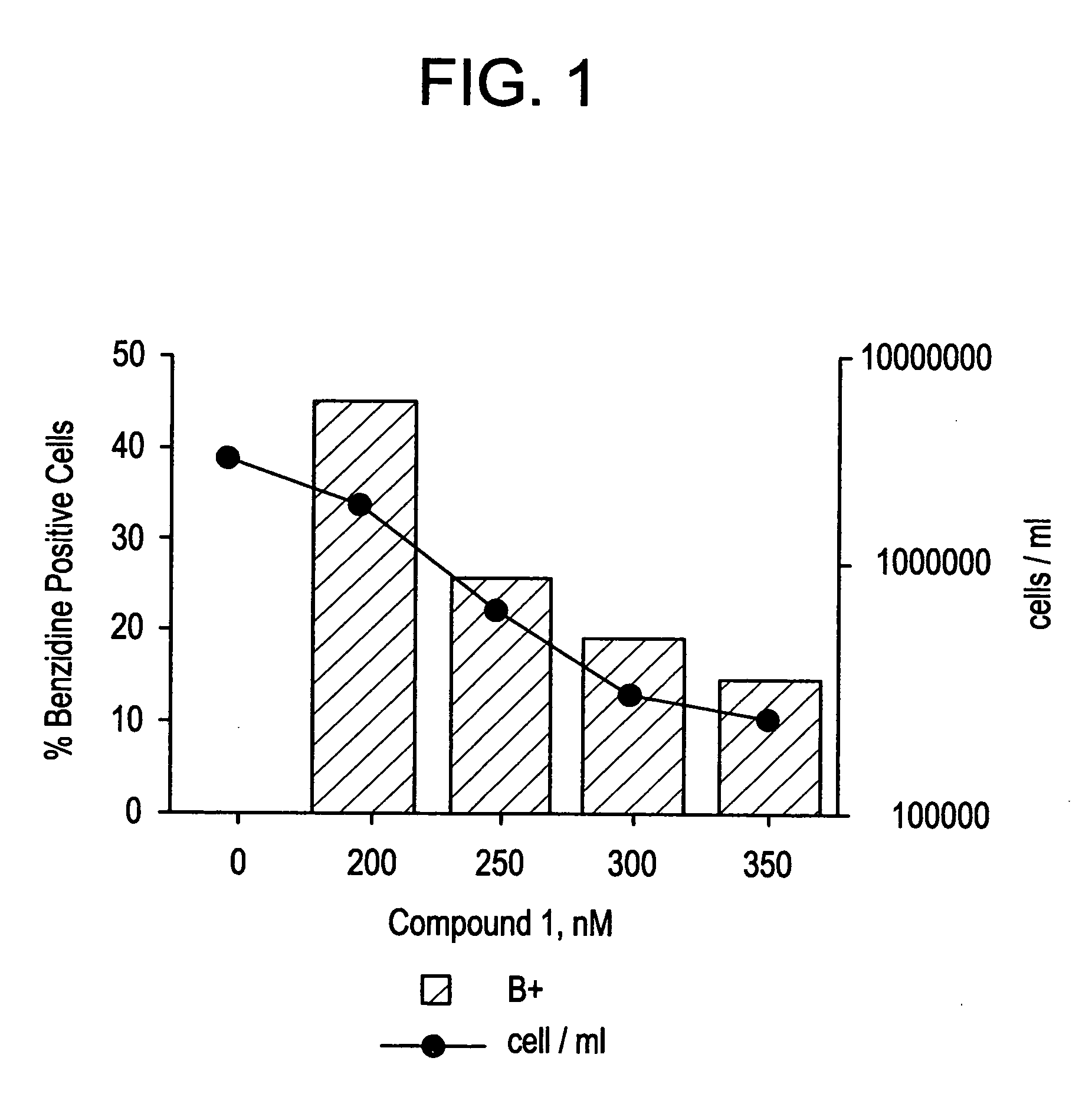
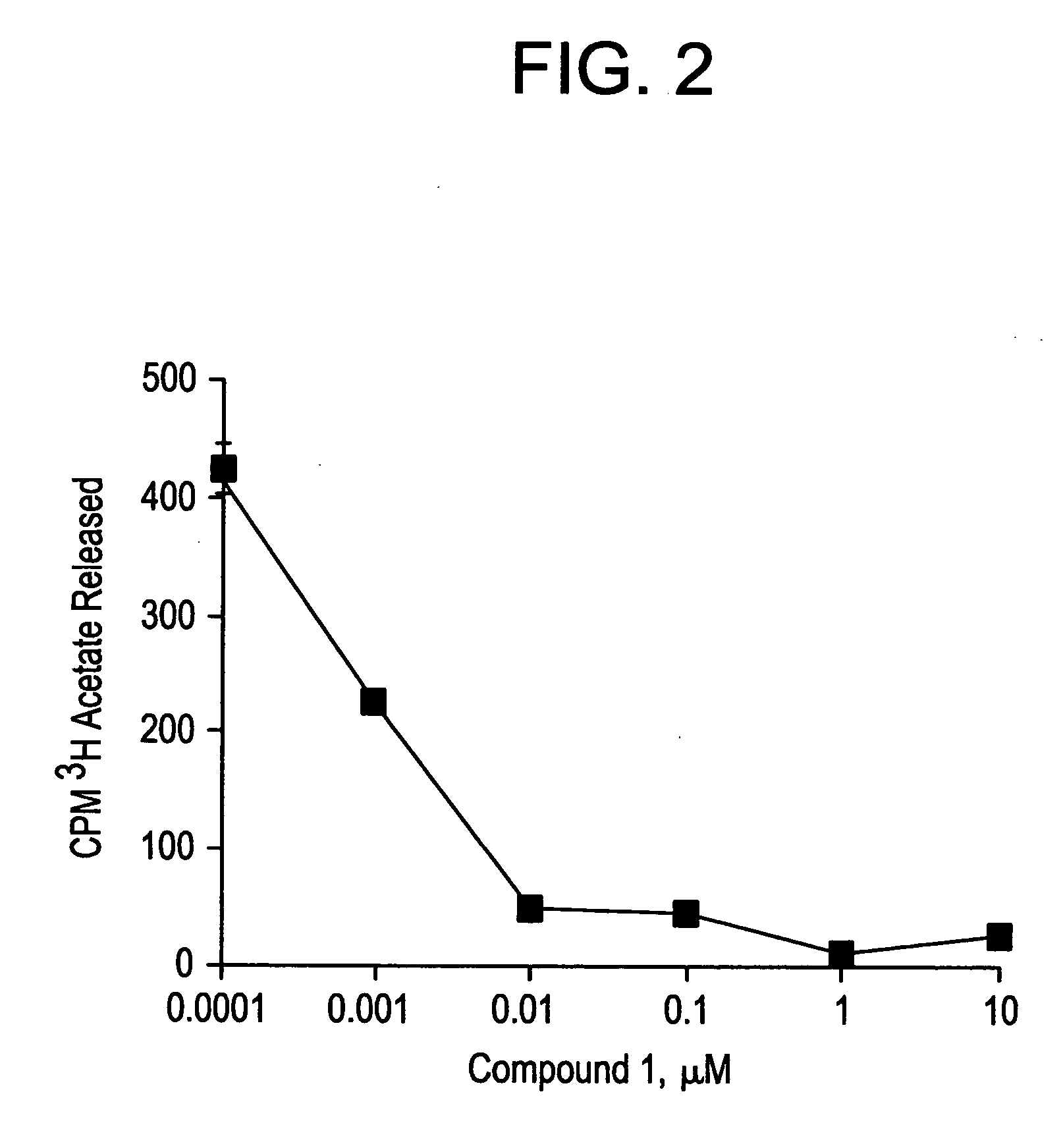
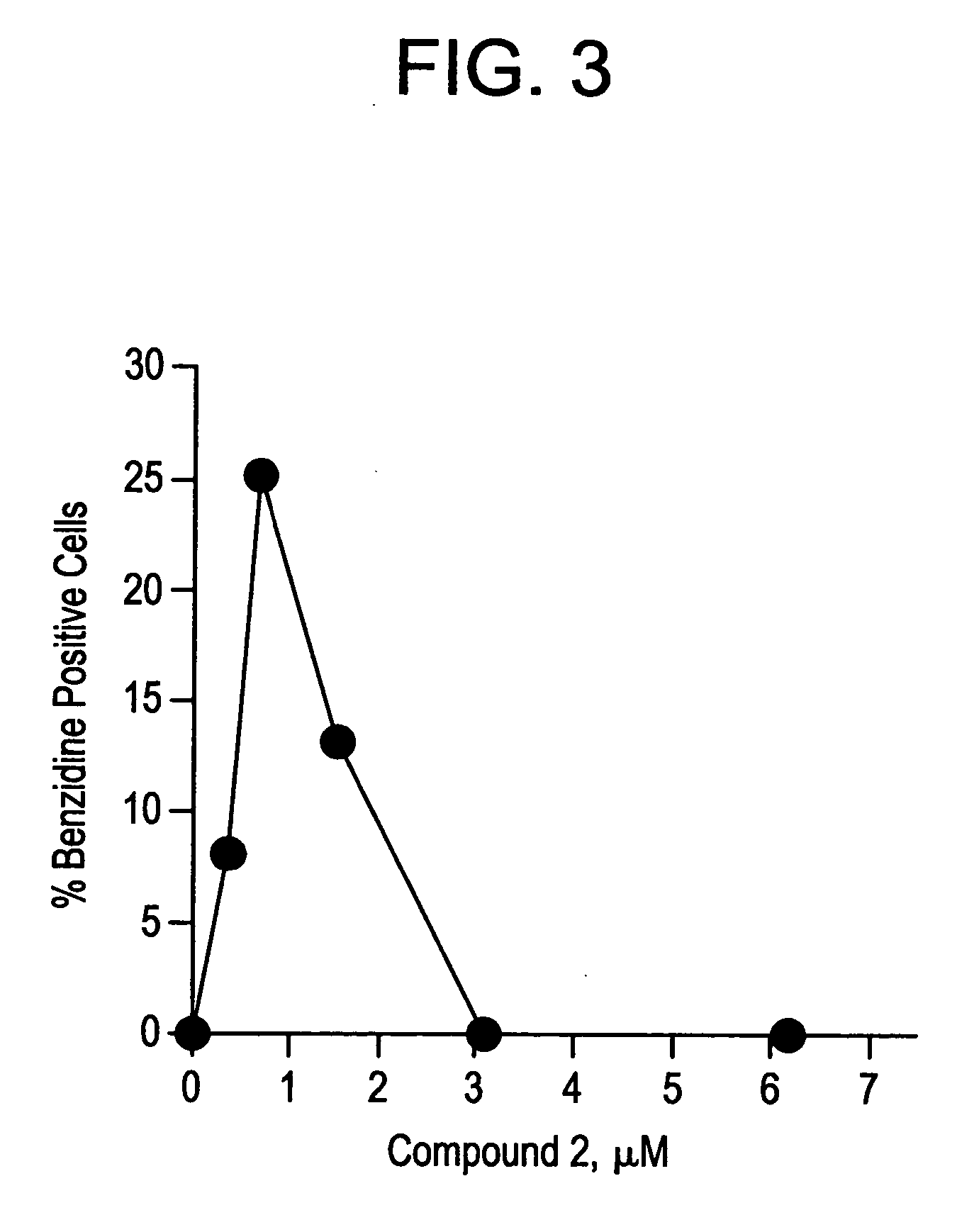
Novel class of cytodifferentiating agents and histone deacetylase inhibitors, and methods of use thereof
a technology of histone deacetylase and cytodifferentiating agent, which is applied in the field of new cytodifferentiating agent and histone deacetylase inhibitors, can solve the problems of limited potency of hmba, and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compound 1
[0086] N-Boc-ω-methyl-(L)-α-aminosuberate, Boc-Asu (OMe) was prepared according to a published procedure (40). (“Boc”=t-butoxycarbonyl; “Asu”=α-aminosuberate (or α-aminosuberic acid))
[0087] N-Cbz-ω-t-butyl-(L)-α-aminosuberate, dicyclohexylamine salt was purchased from Research Plus, Bayonne, N.J.
N-Boc-ω-methyl-(L)-α-aminosuberateanilide, Boc-Asu(OMe)-NHPh
[0088]
[0089] N-Boc-ω-methyl-(L)-α-aminosuberate (493 mg, 1.63 mmoles) was dissolved under Ar in 7 mL of dry CH2Cl2. EDC (470 mg, 2.45 mmoles) was added, followed by aniline (230 μL, 2.52 mmoles). The solution was stirred at room temperature for 2 h 30 min, then washed with dilute HCl (pH 2.4, 2×5 mL), sat. NaHCO3 (10 mL), and H2O (2×10 mL). The product was purified by column chromatography (Silica gel, Hexanes:AcOEt 3.5:1). The isolated yield was 366 mg (60%).
[0090]1H-NMR and Mass Spectroscopy were consistent with the product.
N-Benzoyl-ω-methyl-(L)-α-aminosuberateanilide, PhCOHN-Asu(OMe)-NHPh
[0091]
[0092...
example 2
Synthesis of Compound 2
N-Nicotinoyl-(L)-α-aminosuberoylanilide-ω-hydroxamic acid, C5H4NCO-Asu (NHOH)—NHPh
[0102]
[0103] It was prepared from N-Boc-ω-methyl-L-α-aminosuberate following the same procedure used for the benzoyl analog. Yields and chromatographic behaviour were comparable.
[0104]1H-NMR (d6-DMSO, 500MHz) δ=10.30 (s, 1H), 10.10 (s, 1H), 9.05 (m, 1H), 8.80 (m, 1H), 8.71 (m, 1H), 8.24 (m, 1H), 7.60 (m, 2H), 7.30 (m, 2H), 7.04 (m, 1H), 4.56 (m, 1H), 1.93 (t, 2H), 1.79 (m, 2H), 1.55-1.30 (m, 6H). ESI-MS: 385 (M+1), 407 (M+Na)
example 3
Synthesis of Compound 3
N-benzyloxycarbonyl-ω-t-butyl-(L)-aminosuberic acid, N-Cbz-(L)-Asu (OtBu)-OH
[0105]
[0106] N-Cbz-(L)-Asu (OtBu)-OH, dicyclohexylamine salt (100 mg, 0.178 mmol) was partitioned between 1 M HCl (5 mL) and EtOAc (10 mL). The organic layer was removed, and the aqueous portion washed with EtOAc (3×3 mL). The organic fractions were combined, washed with brine (1×2 mL), and dried (MgSO4). The mixture was filtered and concentrated to a colorless film (67 mg, 0.176 mmol, 99%). This compound was used immediately in the next step.
N-benzyloxycarbonyl-ω-t-butyl-(L)-α-aminosuberateanilide, N-Cbz-(L)-Asu (OtBu)-NHPh
[0107]
[0108] N-Cbz-(L)-Asu (OtBu)-OH (67 mg, 0.176 mmol) was dissolved in dry CH2Cl2 (2.5 mL). Aniline (17 μL, 0.187 mmol), PyBOP (97 mg, 0.187 mmol), and iPr2NEt (46 μL, 0.266 mmol) were added and the mixture stirred for 2 h. The reaction was complete as indicated by TLC. The mixture was diluted with EtOAc (5 mL) and water (5 mL), and the layers separated. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com