Process for preparing 3-heteroaryl-3-hydroxypropanoic acid derivatives
a technology of enantiomer and heteroaryl, which is applied in the field of preparing enantiomerenriched 3heteroaryl3hydroxypropanoic acid derivatives and 3heteroaryl1aminopropan3ols, can solve the problems of inability to exhibit undesirable side effects, inactive or less active enantiomers, and the total yield is economically unacceptabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 and 2
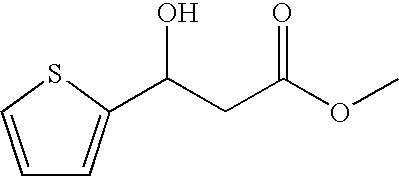
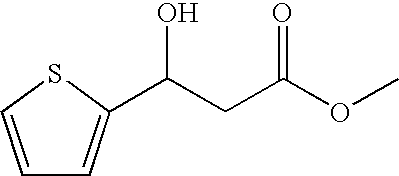
Reduction of methyl 3-oxo-(2-thiophenyl)propanoate Using Different Strains of the Baker's Yeast Saccharomyces cereviseae
[0121] The yeast strains (Saccharomyces cereviseae NG 247, Uniferm GmbH & Co KG, Monheim; Saccharomyces cereviseae Y278, Deutsche Hefe Werke [German Yeast Works] GmbH & Co oHG, Hamburg) were grown overnight, at 28° C. and with shaking (200 rpm), in 100 ml Erlenmeyer flasks containing 25 ml of YM medium (yeast extract, 3.0 g / l; malt extract, 3.0 g / l; peptone, 5.0 g / l; glucose, 10.0 g / l).
[0122] Each of the two yeast strains was incubated, at 28° C. and while shaking (200 rpm), in three 1-litre Erlenmeyer flasks which contained 200 ml of YM medium which had previously been inoculated with 12 ml of preliminary culture. Growth was monitored by measuring the optical density at 600 nm (OD600). After 6-7 h, the cultures reached an OD600 of 3 and were harvested by centrifugation (15 min, 8 000×g) and stored overnight at 4° C. in a refrigerator. For the reaction, 250 μl of...
example 3
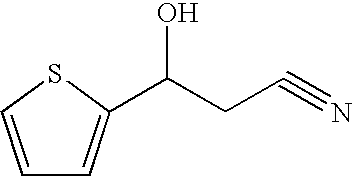
Reduction of 3-(2-thiophenyl)-3-oxopropano-1-nitrile Using Saccharomyces cereviseae Y278
[0123] The yeast cells were grown, and the reaction was carried out, as described in Examples 1 and 2. The result is recorded in Table 2.
TABLE 2StartingReactionYield ofExampleYeast straincompound conc.time [h]Productproductee (S)3Y27820 mM922%85%
examples 4 and 5
Reduction of methyl 3-(2-thiophenyl)-3-oxopropanoate Using Geotrichum candidum
[0124] 200 ml of YM medium were inoculated, in a 1-litre Erlenmeyer flask and as the 1st preliminary culture, with the strain Geotrichum candidum ATCC34614 and the flasks were incubated at 28° C. for 18 h while being shaken. As the 2nd preliminary culture, two 1-litre Erlenmeyer flasks, in each case containing 200 ml of GC medium (KH2PO4, 11.18 g / l; K2HPO4, 3.12 g / l; glycerol, 30.0 g / l; yeast extract, 10.0 g / l; polypeptone, 5.0 g / l), were in each case inoculated with 10 ml of the 1st preliminary culture and likewise shaken at 28° C. for 18 h.
[0125] As the main culture, a 10 litre fermenter was loaded with 4.6 litres of GC medium and inoculated with 400 ml of the 2nd preliminary culture. The culture was grown at 28° C. using an aeration rate of 10 l / min and a stirring rate of 800 rpm. After 10 h, the fermenter was harvested. The cells were sedimented by being centrifuged for 15 min at 6000×g and were then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com