Cycloolefin copolymer formed by ring-opening polymerization, process for producing the same, and optical material
a cycloolefin copolymer and polymerization technology, applied in the direction of optics, instruments, optical elements, etc., can solve the problems of difficult to impart functions such as adhesive properties to the resulting copolymer, difficult to introduce crosslinking groups such as hydrolyzable silyl groups, and difficulty in cycloolefin addition copolymers to achieve the effects of low transparency, high affinity for other materials, and excellent optical properties such as transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0185] A 300-ml glass-made pressure bottle was charged with, under a nitrogen atmosphere, 80 ml of toluene as a solvent, 51 mmol of tricyclo[5.2.1.02.6]-dec-8-ene as a specific monomer (A), in which a molar ratio of an endo form to an exo form was 95:5, 119 mmol of 8-methyl-8-methoxycarbonyltetracyclo[4.4.0.12.5.17.10]dodec-3-ene as a specific monomer (B) and 42.5 mmol of 1-hexene as a molecular weight modifier, and 0.119 mmol of triethylaluminum and 0.017 mmol of a methanol-modified product of tungsten hexachloride [methanol / tungsten=3 (mol / mol)] were further added in this order as a polymerization catalyst. The specific monomer (A) and specific monomer (B) were subjected to ring-opening polymerization at 80° C. for 2 hours, and the polymerization reaction was then terminated by methanol. The conversion of the monomers into a ring-opened copolymer was 97%.
[0186] After 660 ml of water and 47.5 mmol of lactic acid were added to the resultant reaction solution. Then the mixture was s...
example 2
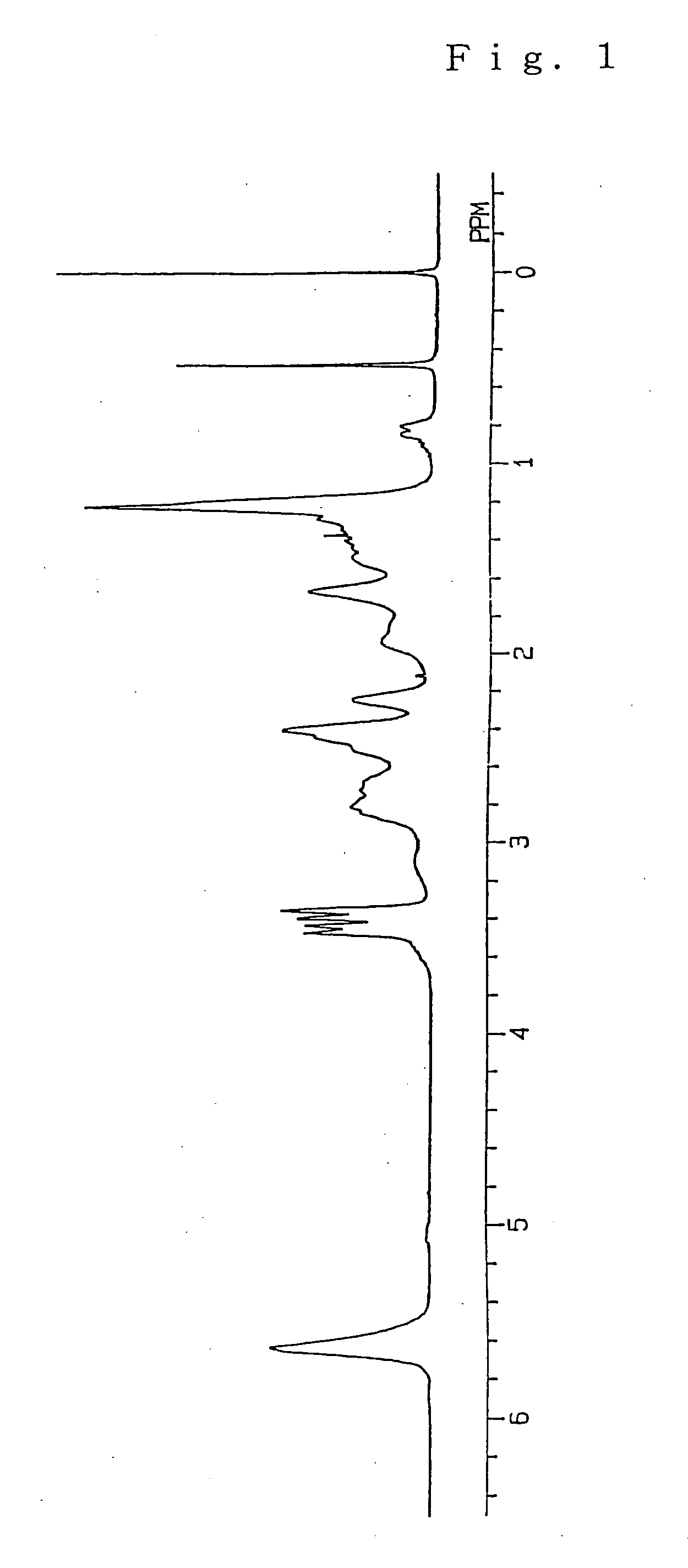
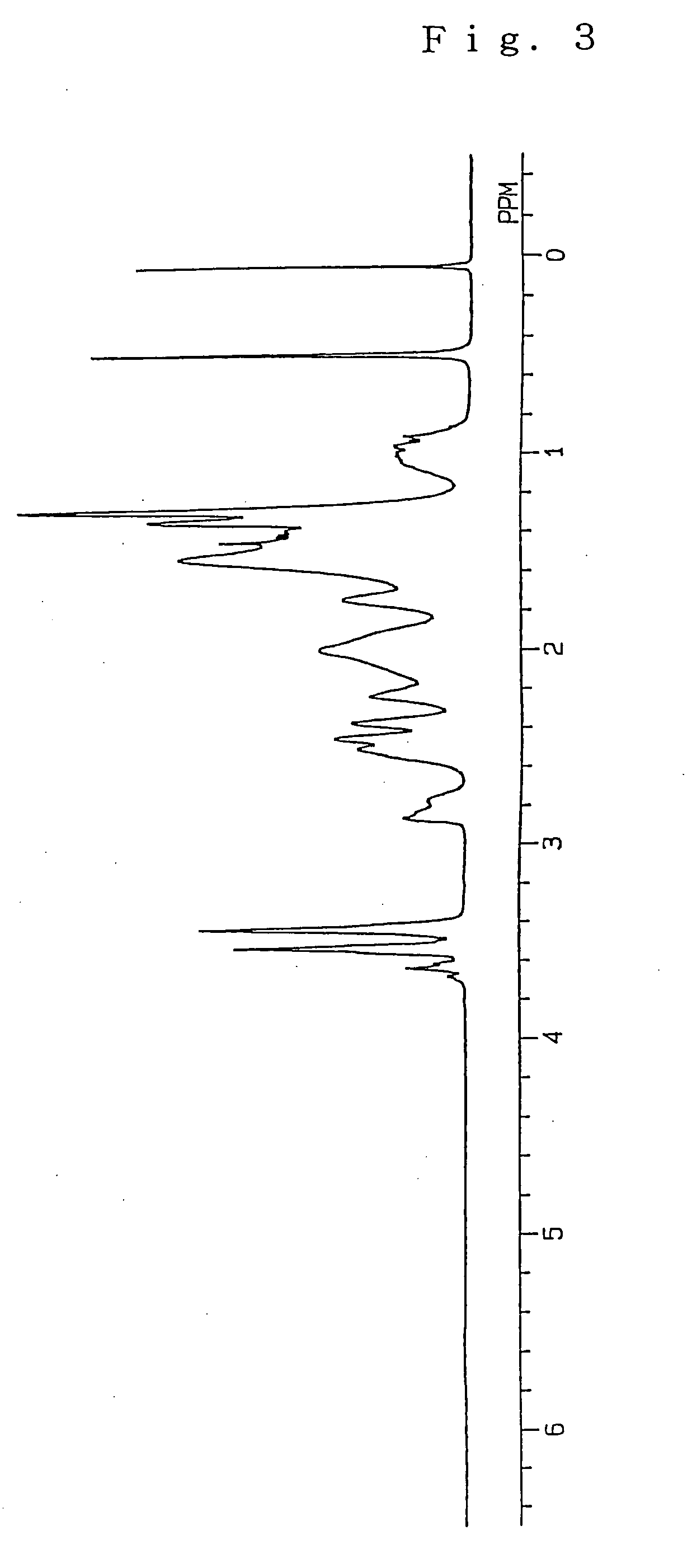
[0191] A ring-opened cycloolefin copolymer (the resultant ring-opened cycloolefin copolymer is referred to as “Ring-Opened Copolymer B”) and a hydrogenated ring-opened cycloolefin copolymer (the resultant hydrogenated ring-opened cycloolefin copolymer is referred to as “Ring-Opened Copolymer BH”) were prepared in the same manner as in Example 1 except that 75 mmol of tricyclo[5.2.1.02.6]dec-8-ene as a specific monomer (A), in which a molar ratio of an endo form to an exo form was 95:5, and 95 mmol of 8-methyl-8-methoxycarbonyl-tetracyclo[4.4.0.12.5.17.10]dodec-3-ene as a specific monomer (B) were used. The conversion of the monomers into the ring-opened copolymer was 90%, and the hydrogenation ratio of Ring-Opened Copolymer BH was 99.8%. The 1H-NMR spectrum of Ring-Opened Copolymer BH is illustrated in FIG. 5.
[0192] A proportion of the structural unit derived from tricyclo[5.2.1.02.6]dec-8-ene in Ring-Opened Copolymer B was 45 mol % (32.3% by weight), and a proportion of the struct...
example 3
[0195] A ring-opened cycloolefin copolymer (the resultant ring-opened cycloolefin copolymer is referred to as “Ring-Opened Copolymer C”) and a hydrogenated ring-opened cycloolefin copolymer (the resultant hydrogenated ring-opened cycloolefin copolymer is referred to as “Ring-Opened Copolymer CH”) were prepared in the same manner as in Example 1 except that tricyclo[5.2.1.02.6]dec-8-ene, in which a molar ratio of an endo form to an exo form was 99:1, was used as a specific monomer (A). The conversion of the monomers into the ring-opened copolymer was 94%, and the hydrogenation rate of Ring-Opened Copolymer CH was 99.7%.
[0196] A proportion of the structural unit derived from tricyclo[5.2.1.02.6]dec-8-ene in Ring-Opened Copolymer C was 32 mol % (21.5% by weight), and a proportion of the structural unit derived from 8-methyl-8-methoxycarbonyltetracyclo-[4.4.0.12.5.17.10]dodec-3-ene was 68 mol % (78.5% by weight).
[0197] The number average molecular weight (Mn) and weight average molecu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com