Soft drink replacer
a soft drink and replacement technology, applied in the field of soft drink replacement, can solve the problems of high phosphoric acid in the soft drink, low water consumption, and poor acceptance of "light" and "diet" soft drinks, and achieve the effects of reducing the risk of cancer, and reducing the effect of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Appetite Reducing Drink
[0098] A unit dosage of an appetite reducing drink containing:
1 320.1 ml water; 4.44 g Genu .RTM. pectin LM-104 AS-Z (Orffa Hercules); 0.08 g CaCO3; 0.02 g Na-saccharine; 0.2 g Na-cyclamate; 0.02 g Sucralose; 0.016 g Carrageenan; 0.44 g Peach-Orange flavor (Quest International); 0.39 g Soy masking (Givaudan); and 0.005 g T-PT8-WS yellow coloring agent (Chr. Hanssen).
[0099] The product has a viscosity of 3.4 mPas at a shear rate of 100 s.sup.-1 at pH 6.02 and at a temperature of 20.degree. C., and a viscosity of at least 1000 mPas at a shear rate of 100 s.sup.-1 at pH 3 and a temperature of 37.degree. C. The caloric density of the product is about 21 kcal per liter.
example 2
Satiety Inducing Effect of Present Drink
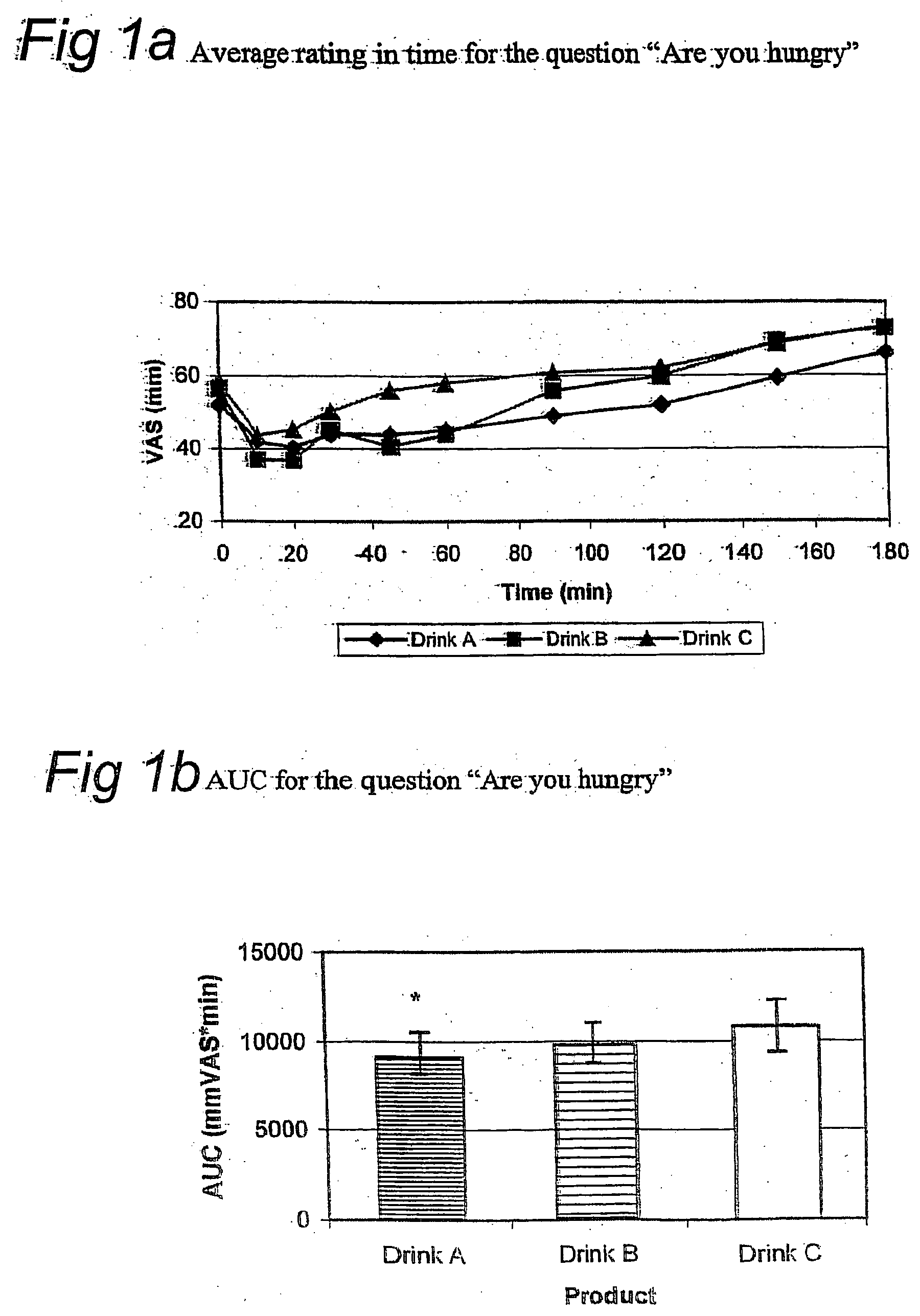
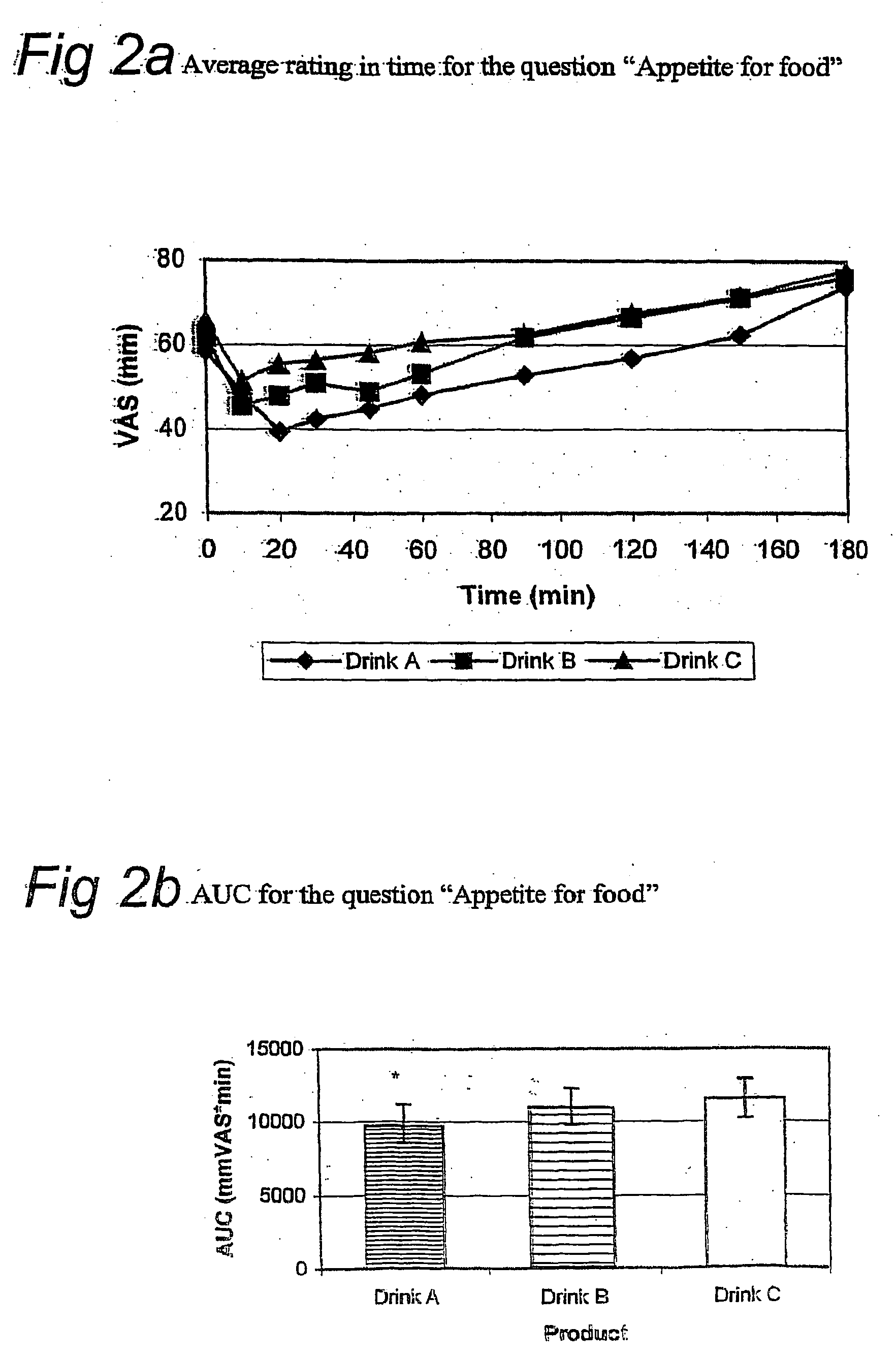
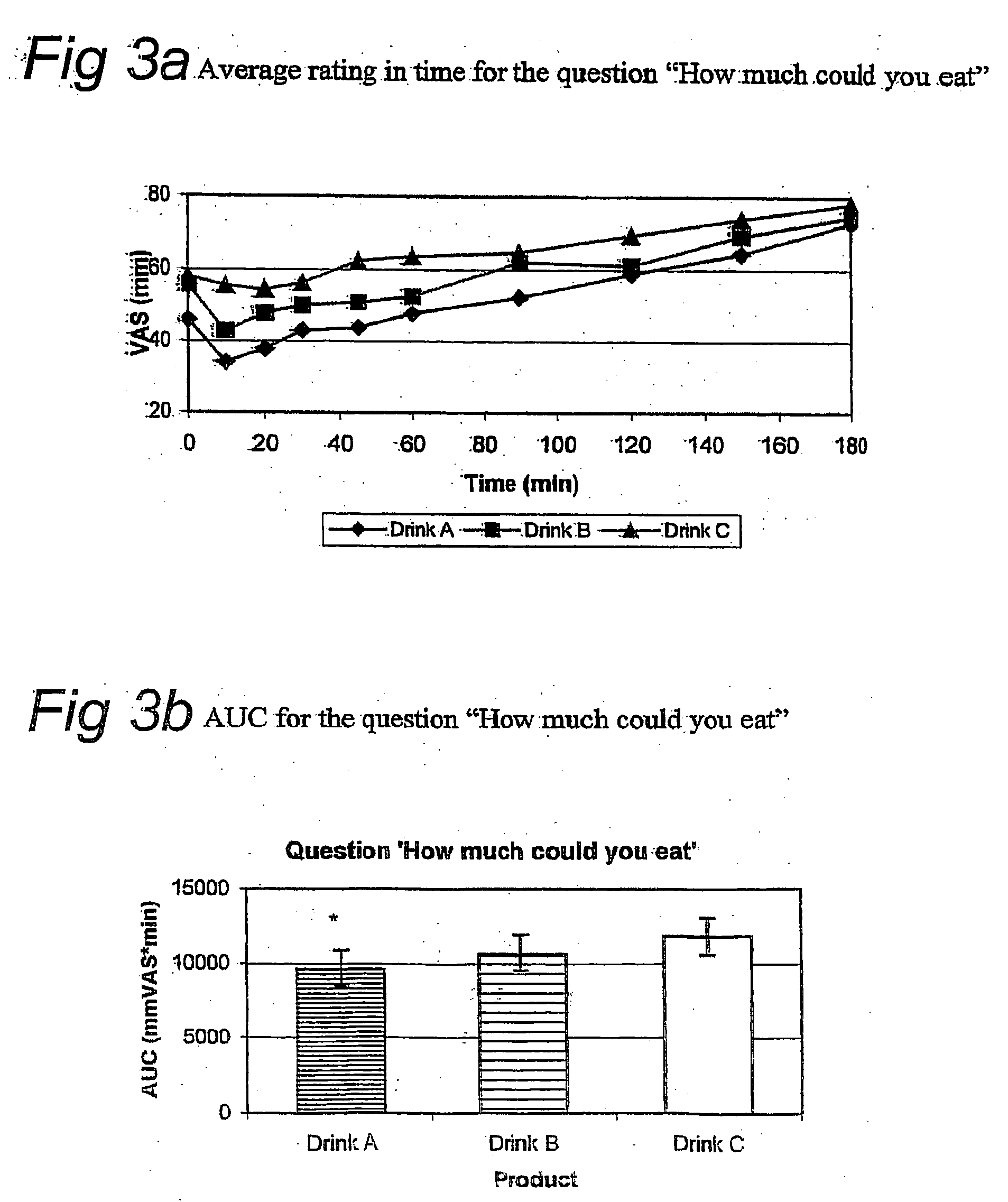
[0100] The appetite suppressive effect and thirst quenching effect of the present drink was tested in humans. In a controlled, blind, randomized cross over study, either one unit dosage of the drink described in example 1 (drink A), one unit dosage of a sugar containing drink (drink B) or one unit dosage of a low caloric drink (drink C) were administered (see Table 1 for ingredients of one unit dosage of the sugar-containing and low-caloric drink). Nine healthy volunteers participated in the study. Subjects ingesting medication that might affect appetite or satiety were excluded.
[0101] Subjects were randomly allocated to one of three study groups. Each group received drink A, B and C on three separate days, but in different order. Group 1 (n=3): drink A on day 1, drink B on day 2, and drink C on day 3; Group 2 (n=3): drink B on day 1, drink C on day 2, and drink A on day 3; Group 3 (n=3): drink C on day 1, drink A on day 2, and drink B on day ...
example 3
Calcium Availability During Fermentation
[0116] Preparation of MacFarlane Medium
3 Buffered peptone water 3.0 g / l Yeast Extract 2.5 g / l Tryptone 3.0 g / l L-Cysteine-HCl 0.4 g / l Bile salts 0.05 g / l K.sub.2HPO.sub.4.3H2O 2.6 g / l NaHCO.sub.3 0.2 g / l NaCl 4.5 g / l MgSO.sub.4.7H.sub.2O 0.5 g / l CaCl.sub.2 0.228 g / l FeSO.sub.4.7H.sub.2O 0.005 g / l
[0117] pH was adjusted to 6.3.+-.0.1 using 2M HCl and subsequently the medium was sterilized.
[0118] Preparation or Fecal Suspension:
[0119] Under anaerobic conditions, human feces were suspended in McFarlane medium in a weight ratio feces: MacFarlane medium of 1:5. The suspension was subsequently sieved to remove solid components.
[0120] Fermentation
[0121] 15 ml of the fecal suspension was mixed with a dry mixture consisting of either pectin and calcium; or pectin, calcium and oligosaccharide (see Tabel 9) and incubated for 24 hours at 37.degree. C. under anaerobic conditions. After incubation, the solids were removed from the suspension by centrifugatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com