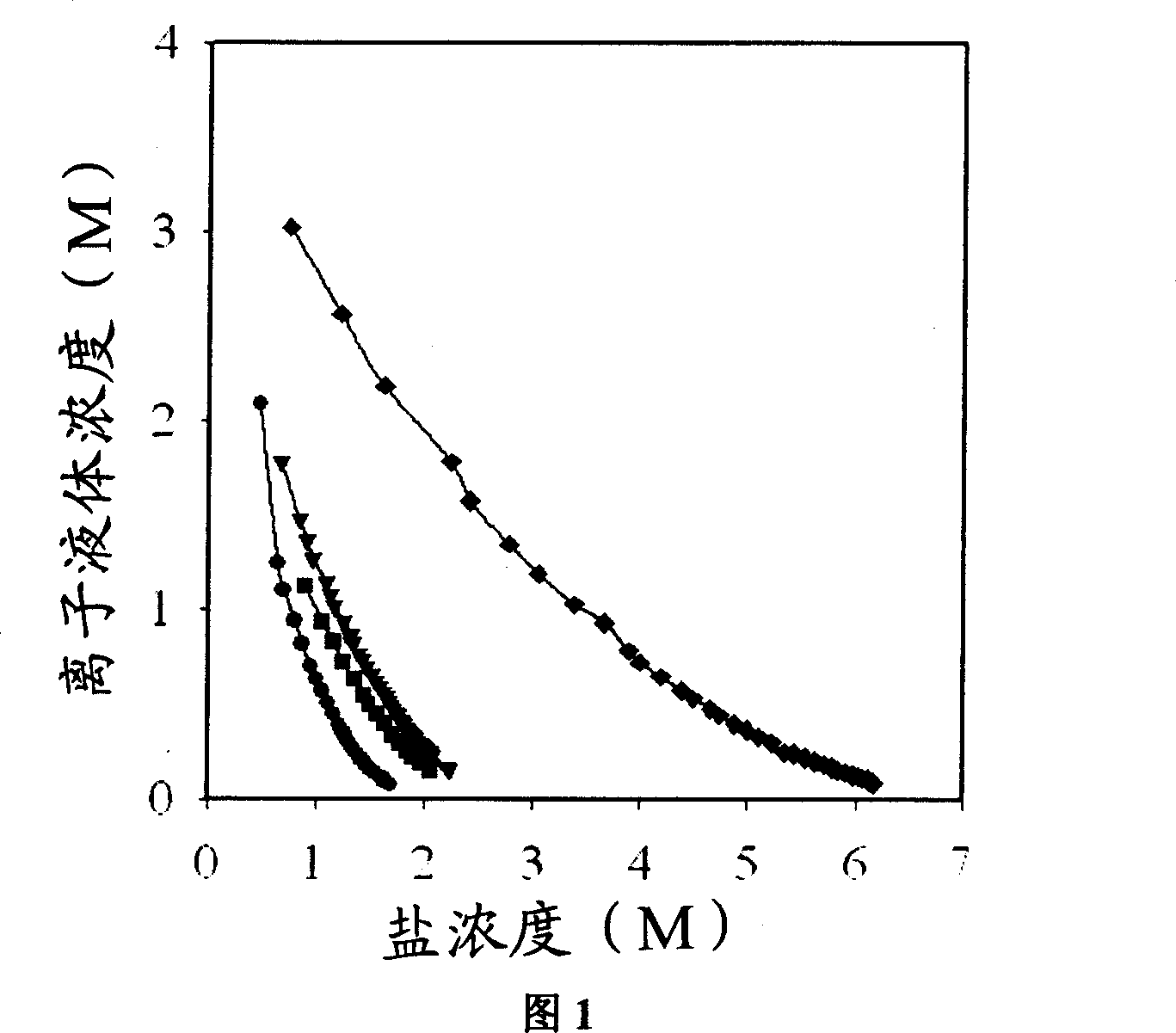
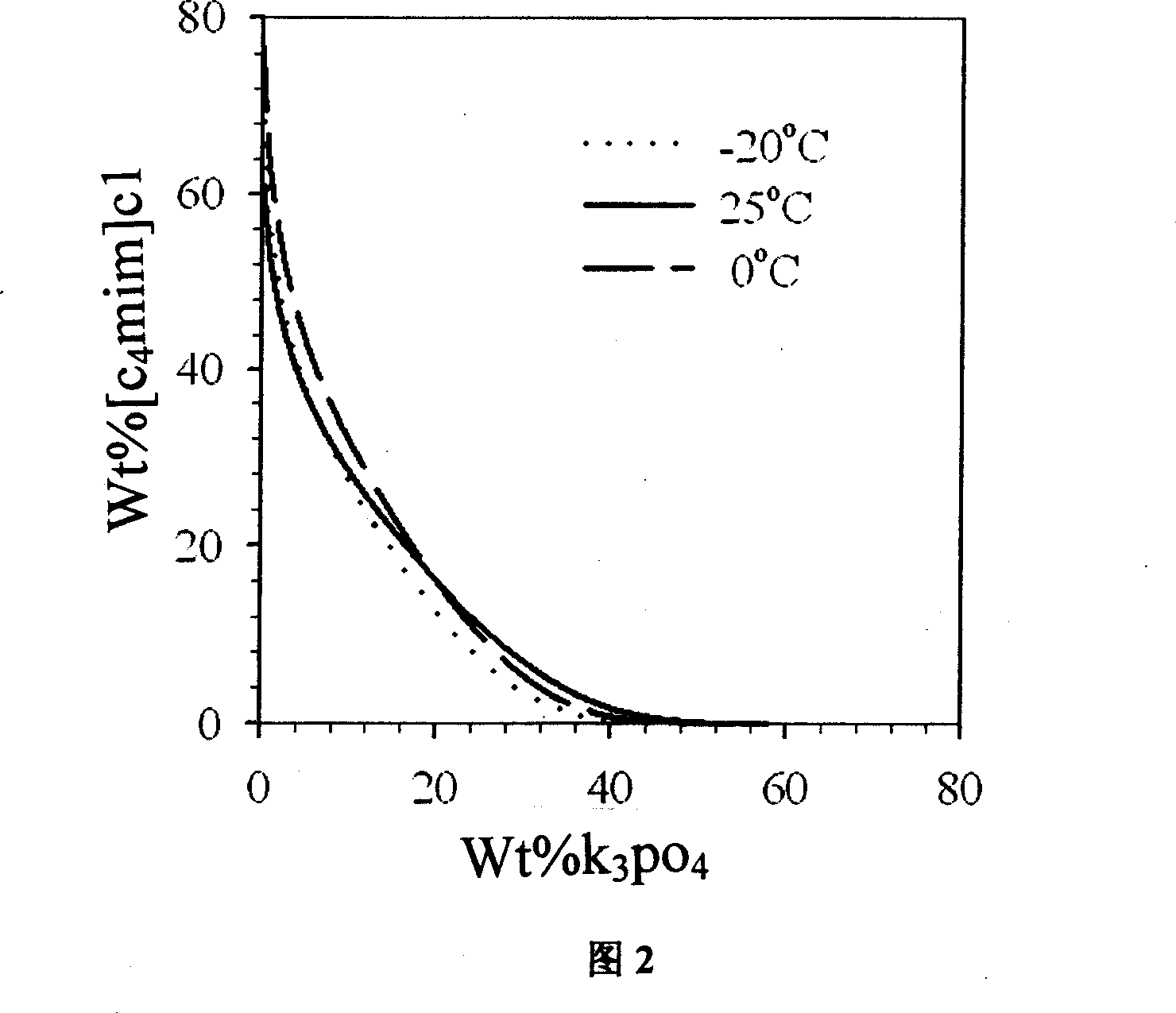
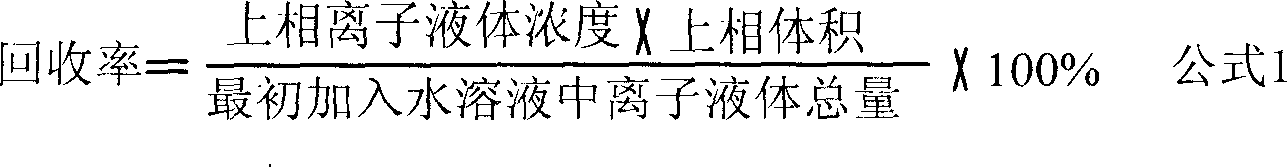
Process of enriching ionic liquid from water solution
A technology of ionic liquid and aqueous solution, which is applied in the field of ionic liquid enrichment technology, can solve the problems of high cost and high energy consumption for removing water, and achieve the effects of low cost, large processing capacity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: K 3 PO 4 Recovery of [C in aqueous solution 4 mim]Cl
[0026] 1) Add 10 g of 10% [C 4 mim]Cl aqueous solution, then add 4g, 5g, 6g, 7g and 8gK 3 PO 4 , the corresponding sample numbers are 1-5 respectively, placed on a vortex mixer and shaken for 5 minutes to dissolve and mix evenly, and stand to form an upper phase and a lower phase two-phase aqueous system; 2) Use a separatory funnel to separate the two phases, Evaporate the upper ionic liquid-rich phase at normal pressure at 60°C for 72 hours to remove water and precipitate the salt crystals contained in the upper phase, then dry it in vacuum at 50°C for 24 hours to obtain pure ionic liquid, recovery of ionic liquid The efficiency results are listed in Table 1; 3) Lower the temperature of the salt-rich phase of the lower phase to 0 °C to form a new two-phase system, and the remaining ionic liquid is enriched in the upper phase, and repeat step 2) to obtain a pure ionic liquid again . Repeat step 3),...
Embodiment 2
[0035] Example 2: K 2 HPO 4 Recovery of [C in aqueous solution 4 mim]Cl
[0036] According to the method of Example 1, but add 5g, 6g, 7g, 8g K 2 HPO 4 , the corresponding sample numbers are 6-9, K 2 HPO 4 Mass pair [C 4 The impact results of mim]Cl recovery are listed in Table 3.
[0037] Numbering
[0038] From Table 3 we can see that when K 2 HPO 4 When the quality of the ionic liquid is 5.0514g and 6.0206g, the recovery rate of the ionic liquid is 83.01% and 95.34%, and the temperature of the lower phase salt-rich phase of the No. 6 and No. 7 samples is lowered to 0 ° C or -20 ° C, and the double water phase is formed. system, adopt the same analysis method, and the results of the determination of recovery are listed in Table 4.
[0039] Numbering
[0040] From Table 4, we can see that the total recovery can reach 85.78% and 97.18% by lowering the temperature to 0°C and adopting two-stage enrichment of ionic liquids; lowering the temperature...
Embodiment 3
[0041] Example 3: K 2 CO 3 Recovery of [C in aqueous solution 4 mim]Cl
[0042] According to the method of Example 1, but adding 4g, 5g, 6g, 7g and 8g K 2 CO 3 , the corresponding sample numbers are 10-14, K 2 CO 3 Mass pair [C 4 The impact results of mim]Cl recovery are listed in Table 5.
[0043] Numbering
[0044] From Table 5 we can see that when K 2 CO 3 When the mass of the ionic liquid was 5.0419g and 6.0614g, the recovery rate of the ionic liquid was 90.96% and 96.59%, and the temperature of the lower salt-rich phase of the No. 11 and No. 12 samples was lowered to 0 ° C or -20 ° C, and the double water phase was formed. system, adopt the same analysis method, and the results of the determination of recovery are listed in Table 6.
[0045] Numbering
[0046] From Table 6, we can see that the total recovery can reach 93.82% and 97.73% by lowering the temperature to 0°C and adopting two-stage enrichment of ionic liquids; lowering the temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com