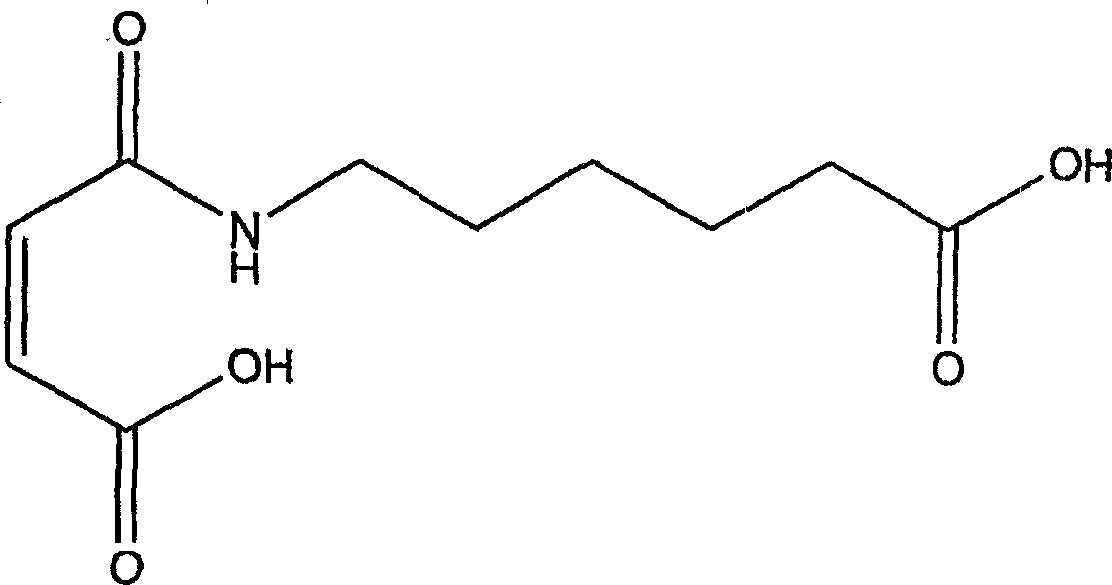
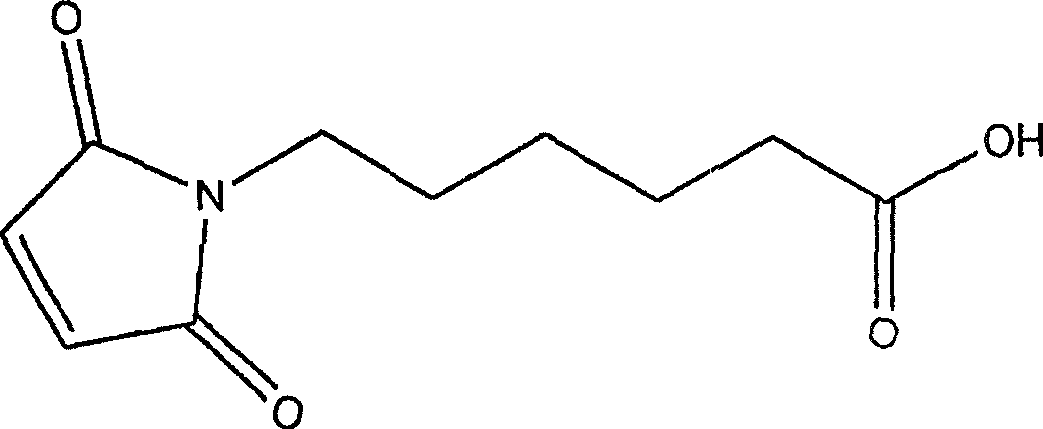
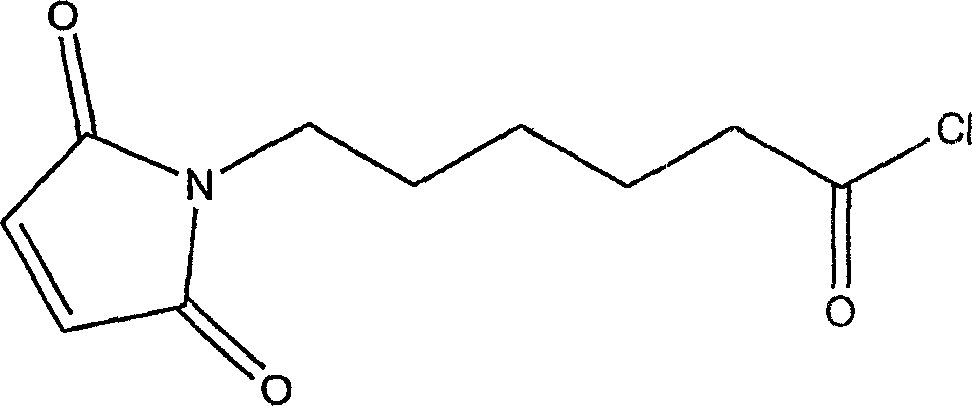
Natural biodegradable polysaccharide coatings for medical articles
A biological, coating technology, applied in the direction of coating, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0235] Synthesis of Acrylated Amylose
[0236] Amylose with polymerizable vinyl groups was prepared by mixing 0.75 g of amylose (A0512; Aldrich) and 100 mL of dimethyl sulfoxide (JT Baker) in a 250 mL amber bottle, under stirring. One hour later, 2 mL of triethylamine (TEA; Aldrich) was added, and the resulting mixture was stirred at room temperature for 5 minutes. Subsequently, 2 mL of glycidyl acrylate (Polysciences) was added, and amylose and glycidyl acrylate were allowed to react overnight with stirring at room temperature. The mixture containing the amylose-glycidyl acrylate reaction product was dialyzed with DI water using continuous flow dialysis for 3 days. The obtained acrylated amylose (0.50 g; yield 71.4%) was lyophilized and stored at room temperature and protected from light.
Embodiment 2
[0238] Synthesis of MTA-PAAm
[0239] The polymerization initiator is prepared by copolymerizing methacrylamide with photosensitive group and acrylamide.
[0240] Firstly, methacrylamide-oxothixanthene monomer (N-[3-(7-methyl-9-oxothioxanthene-3-formylamino) propyl] methacrylamide (MTA-APMA) is prepared ). In a 250mL round bottom flask equipped with a drying tube, 4.53g (25.4mmol) of N-(3-aminopropyl)methacrylamide hydrochloride (APMA) prepared according to the method described in Example 2 of US Patent No. 5,858,653 ), suspended in 100mL anhydrous chloroform. 7-Methyl-9-oxothixanthene-3-carboxylic acid (MTA) was prepared according to the method described in Example D of US Patent No. 4,506,083. MTA-chloride (MTA-Cl) was prepared according to the method described in Example 1 of US Patent No. 6,007,833. After cooling the slurry in an ice bath, solid MTA-Cl (7.69 g; 26.6 mmol) was added to the APMA-chloroform suspension under stirring. Then, a solution containing 7.42 mL...
Embodiment 3
[0243] Formation of amylose coating
[0244] 100 mg of the acrylated amylose prepared in Example 1 was placed in an 8 mL amber bottle. Add 3 mg MTA-PAAm (lyophilized), 2 μL of 2-NVP (N-vinyl-2-pyrrolidone; accelerator (Bimax)) and 1 mL of 1X phosphate buffered saline ( 1X PBS). The reagents were then mixed on a shaker at 37°C for 1 hour. Place 50μL of the mixture on a glass slide (2991FI; Esco), and use a 400-500nm filter (50mW / cm 2 ) The EFOS 100 SS lighting system is illuminated for 50 seconds. After exposure to light, the polymer was found to form a semi-hard gel with elastomer properties.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com