Novel bichalcophenes and their prodrugs as antiprotozoal agents
A C2-C10, pharmaceutical technology, applied in anti-infective drugs, anti-infective drugs, and resistance to vector-borne diseases, etc., can solve serious side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
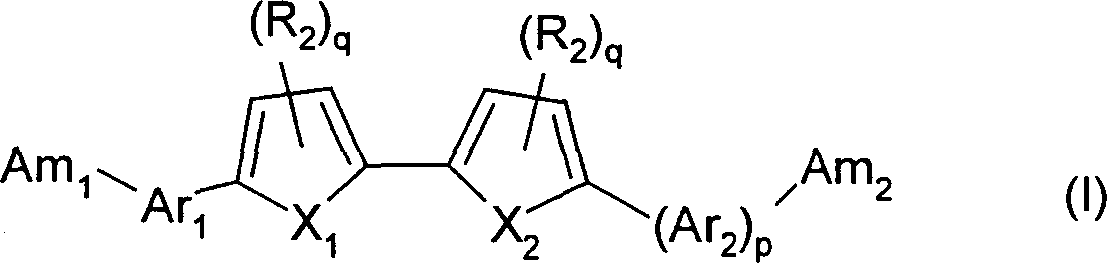
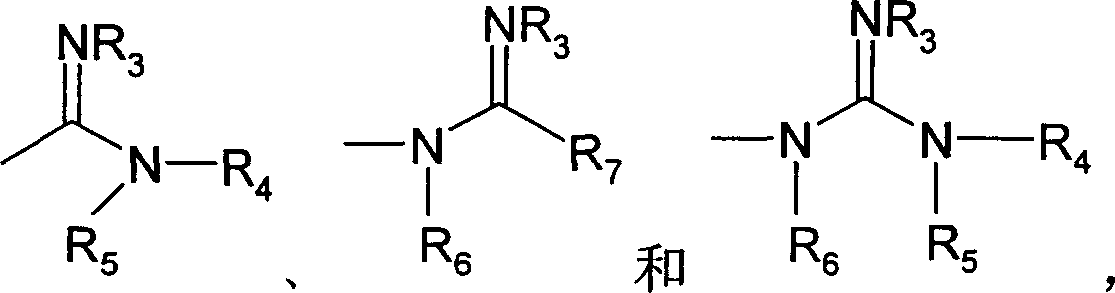
[0344] In some embodiments, the present invention provides a method for preparing a compound of formula (I) and a pharmaceutically acceptable salt thereof, the method comprising:
[0345] (a) contacting a first cyano-substituted heterocyclic compound with N-bromosuccinimide to form a first brominated heterocyclic compound;
[0346] (b) coupling said first brominated heterocyclic compound with a second heterocyclic compound to form a third heterocyclic compound;
[0347] (c) reacting the third heterocyclic compound with one of the following:
[0348] (i) reacting with a strong acid and anhydrous alcohol, followed by reaction with ammonia and anhydrous alcohol, to form a compound of formula (I), wherein the compound of formula (I) is a bisamidine;
[0349] (ii) react with hydroxylamine hydrochloride and base, form the compound of formula (I), wherein the compound of said formula (I) is bisamidoxime; With
[0350] (iii) reacting with lithium bis(trialkylsilyl)amide for a period...
Embodiment 1
[0404] 5'-(4-amidinophenyl)-2,2'-bifuran-5-carboxamidine
[0405]
[0406] Reagents and conditions: (i) NBS, DMF; (ii) 2-tributyltinfuran, Pd(PPh 3 ) 4 ; (iii) CuCN, DMF, 110-120 ° C; (iv) NH 2 OH·HCl, KO-t-Bu, DMSO; (v) AcOH / Ac 2 O; (vi)H 2 / Pd-C, AcOH.
[0407] Synthesis of route 2.5'-(4-amidinophenyl)-2,2'-bifuran-5-carboxamidine
[0408] 4-(5-Bromofuran-2-yl)-benzonitrile (2). Now referring to route 2 above, to 1 (8.45 g, 50 mmol) in DMF (30 ml) was added N-bromo Subsuccinimide (9.79 g, 55 mmol). The reaction mixture was stirred overnight, then poured onto cold water. The formed precipitate was collected, washed with water, and dried to give analytically pure product 2 in 94.2% yield, mp 94-94.5 °C. 1 H NMR (CDCl 3 ): δ6.45(d, J=3.6 Hz, 1H), 6.76(d, J=3.6 Hz, 1H), 7.66(d, J=8.4Hz, 2H), 7.70(d, J=8.4Hz, 2H ). 13 C NMR (CDCl 3 ): δ153.7, 133.5, 132.6, 123.8, 123.6, 118.7, 114.0, 110.7, 110.3. EIMS (m / z, rel.int.): 247 (M + , 50), 140(100), 113(10). C 11 h ...
Embodiment 2
[0417] 6-(5'-amidino-2,2'-bifuran-5-yl)-nicotinamide
[0418] (6-(5'-Amidino-2,2'-bifuran-5-yl)-nicotinamidine)
[0419]
[0420] Reagents and conditions: (i) 2-tributyltinfuran, Pd(PPh 3 ) 4 ; (ii) NBS, DMF; (iii) CuCN, DMF110-120 ° C; (iv) NH 2 OH·HCl, KO-t-Bu, DMSO; (v)a) AcOH / Ac 2 O; b) H 2 / Pd-C,A c Oh.
[0421] Route 3. Synthesis of 6-(5'-amidino-2,2'-bifuran-5-yl)-nicotinamide
[0422] 6-(2,2'-Bifuran-5-yl)-nicotinonitrile (10). Referring now to Scheme 3, start from 9 using the same procedure as described for 3. Yield 78%, mp 169-170°C. 1 H NMR (CDCl 3 ): δ6.52(dd, J=3.6Hz, J=1.8Hz, 1H), 6.74(m, 2H), 7.31(d, J=3.6Hz, 1H), 7.49(d, J=1.8Hz, 1H ), 7.79(d, J=8.4Hz, 1H), 7.95(dd, J=8.4, 2.1Hz, 1H), 8.80(d, J=2.1Hz, 1H). 13 C NMR: δ152.5, 151.3, 151.0, 148.7, 145.5, 142.9, 139.6, 117.6, 117.0, 114.3, 111.7, 108.1, 107.1, 106.6. C 14 h 8 N 2 o 2 Calculated: C, 71.18; H, 3.41; N, 11.85. Found: C, 70.83; H, 3.61; N, 11.84.
[0423] 6-(5'-Bromo-2,2'-bifuran...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap