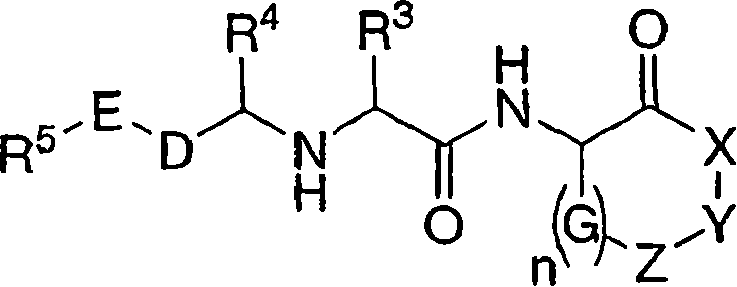
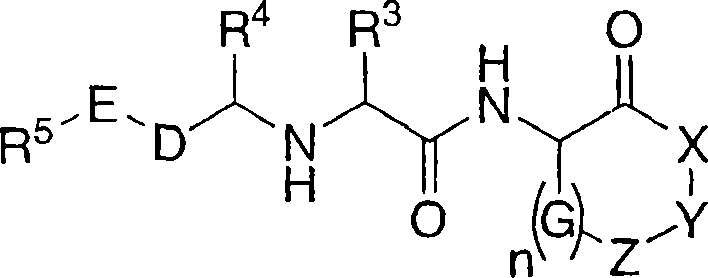
Cathepsin cysteine protease inhibitors
A compound, alkyl technology, applied in the field of cathepsin cysteine protease inhibitors, can solve problems such as inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0218] N 1 -(2-oxotetrahydrofuran-3-yl)-N 2 -{(1S)-2,2,2-Trifluoro-1-[4'-(methylsulfonyl)biphenyl-4-yl]ethyl}-L-leucinamide
[0219]
[0220] Under stirring at room temperature within 30 minutes, to N-{(1S)-2,2,2-trifluoro-1-[4'-(methylsulfonyl)biphenyl-4-yl]ethyl}-L - Leucine (30 mg, 68 μmol), benzotriazol-1-yl-oxotripyrrophosphine hexafluorophosphate (40 mg, 77 μmol) and α-amino-γ-butyrolactone (26 mg, 140 μmol) in DMF (0.20ml) was added triethylamine (30μl, 220μmol). The mixture was partitioned between ethyl acetate and water and the layers were separated. The organic phase was washed with brine, dried (sodium sulfate) and concentrated, and purified by flash chromatography on silica gel eluting with ethyl acetate to afford the title compound as a colorless solid. MS(+ESI):527.2(M+1) + .
[0221] h 1 NMR (acetone-d 6 , 500MHz) δ8.06 (4H, d), 8.00-7.95 (4H, m), 7.90 (1H, m), 7.83 (1H, overlapping m), 7.80 (4H, d), 7.67 (4H, t), 4.58(2H,m), 4.45(2H,m), 4.37(2H,m),...
Embodiment 2
[0223] N 1 -[2-Oxo-1-(phenylsulfonyl)pyrrolidin-3-yl]-N2 -{(1S)-2,2,2-Trifluoro-1-[4'-(methylsulfonyl)biphenyl-4-yl]ethyl}-L-leucinamide
[0224]
[0225] Under stirring at room temperature within 2.5 hours, to N-{(1S)-2,2,2-trifluoro-1-[4'-(methylsulfonyl)biphenyl-4-yl]ethyl}-L -Leucine (40 mg, 90 μmol), benzotriazol-1-yl-oxotripyrrophosphine hexafluorophosphate (55 mg, 110 μmol) and (54 mg, 220 μmol) in DMF (0.2ml) were added tris Ethylamine (30 μl, 220 μmol). The mixture was partitioned between ethyl acetate and water and the layers were separated. The organic phase was washed with brine, dried (sodium sulfate) and concentrated, and purified by flash chromatography on silica gel eluting with 3 / 2 ethyl acetate / hexanes to give the title compound as a colorless thick syrup. MS(+ESI):666.3(M+1) + .
[0226] h 1 NMR (acetone-d 6 , 500MHz) δ8.07-7.97 (8H, d), 7.96-7.92 (4H, m), 7.86-7.70 (8H, m), 7.65-7.57 (8H, m), 4.54 (2H, m), 4.40 ( 2H, m), 3.93 (2H, m), 3.80 (2H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com