Use of CETP inhibitors and optionally HMG COA reductable inhibitors and/or antihypertensive agents
A drug, selected technology, applied in the field of elaide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0187] The present invention is not limited to any specific structure or type of CETP inhibitor. In contrast, the present invention has general suitability for a class of CETP inhibitors. The compounds that are the subject of the present invention can be found in many patents and published applications, including: DE19741400A1; DE19741399A1; WO9914215A1; WO9914174; DE19709125A1; DE19704244A1; DE19704243A1; EP818448A1; WO9804528A2; WO9941237A1; WO9914204A1; WO9835937A1; JP11049743; WO200018721; WO200018723; WO200018724; WO200017164; WO200017165; WO200017166; EP992496; and EP987251, the entire contents of these documents are incorporated herein by reference for all purposes.
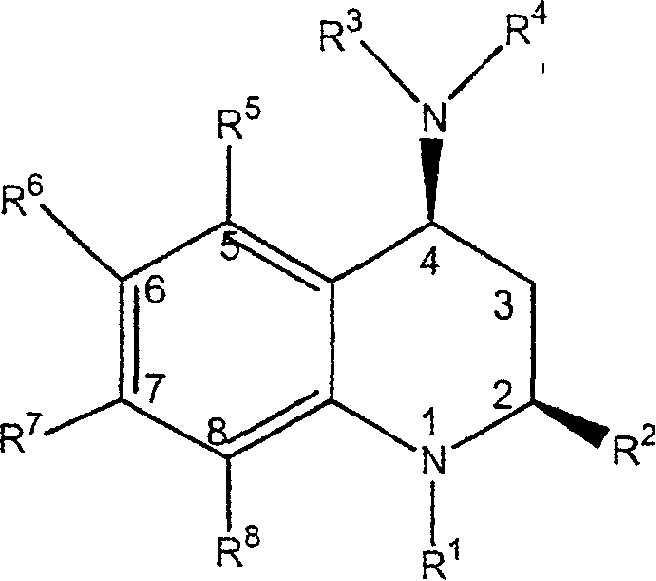
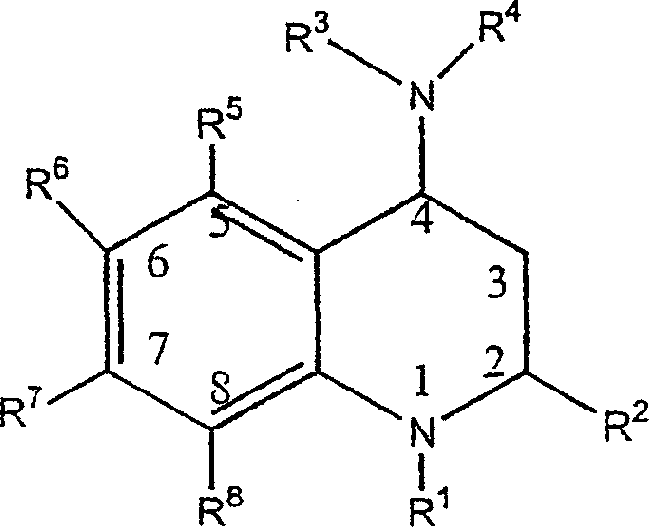
[0188] A class of CETP inhibitors used in the present invention are 4-carboxyamino-2-methyl-1,2,3,4-tetrahydroquinolines substituted with oxygen having the general formula I and the compounds are pharmaceutically acceptable The salt, enantiomer or stereoisomer composition of:
[0189]
[0190] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com