Treatment with anti-ErbB2 antibodies
An antibody and a technology for use in the field of treating diseases characterized by overexpression of ErbB2, which can solve problems such as stimulating tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0154] Materials and methods
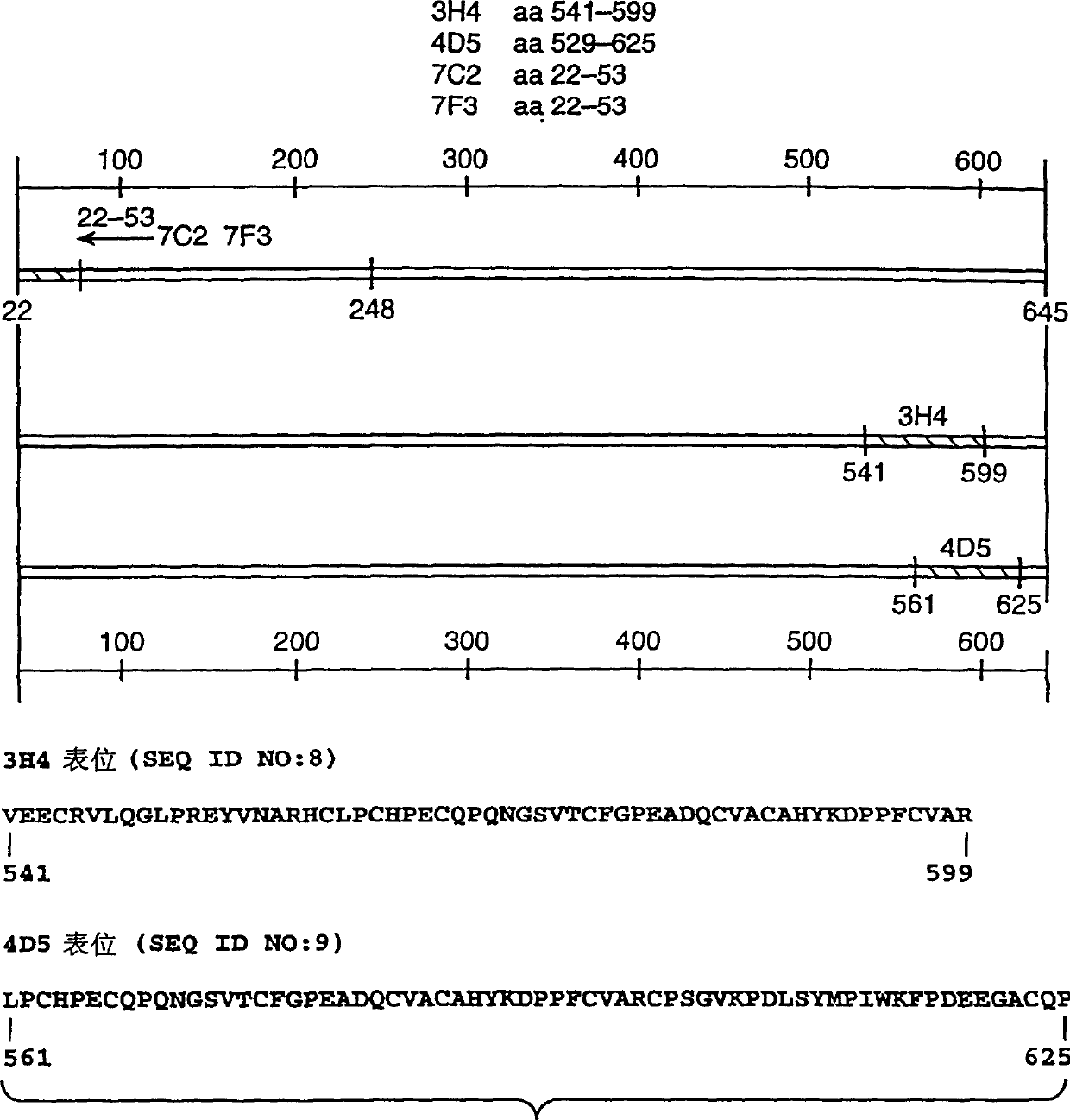
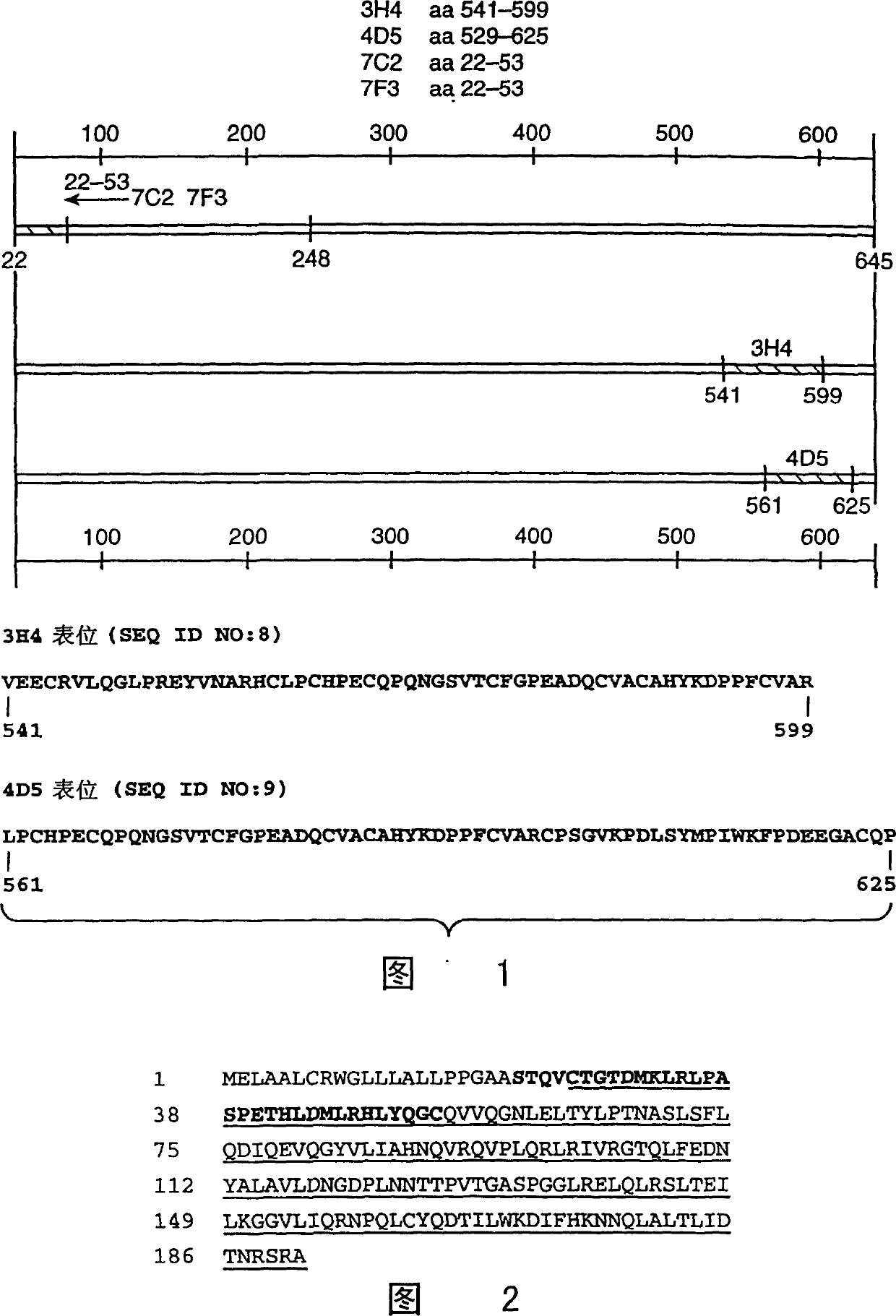
[0155] Anti-ErbB2 monoclonal antibody. Anti-ErbB2 IgG specific for the ErbB2 extracellular domain was produced as described by Fendly et al. Cancer Res. 50:1550-1558 (1990) and WO89 / 06692 1 Kappa mouse monoclonal antibody 4D5. Briefly, NIH3T3 / HER2-3 was generated as described by Hudziak et al., Proc. Natl. Acad. Sci. (USA) 84:7159 (1987) 400 cells (each cell expresses about 1×10 5 ErbB2 molecules), the cells were harvested in phosphate buffered saline (PBS) containing 25 mM EDTA and used to immunize BALB / c mice. At the 0th, 2nd, 5th, and 7th weeks, the mice were intraperitoneally injected with 10 7 Cells in 0.5 ml PBS. At weeks 9 and 13, mice whose antiserum could immunoprecipitate 32p-labeled ErbB2 were injected intraperitoneally with WGA-purified ErbB2 membrane extracts. Then, 0.1 ml of the ErbB2 preparation was injected intravenously once, and the splenocytes were fused with the mouse myeloma cell line X63-Ag8.653. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com