Methods of treatment with virus-based gene therapy
A gene therapy, virus technology, applied in the field of treatment with virus-based gene therapy, can solve problems such as breakthrough bleeding, negative impact on daily life, and inconvenient cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
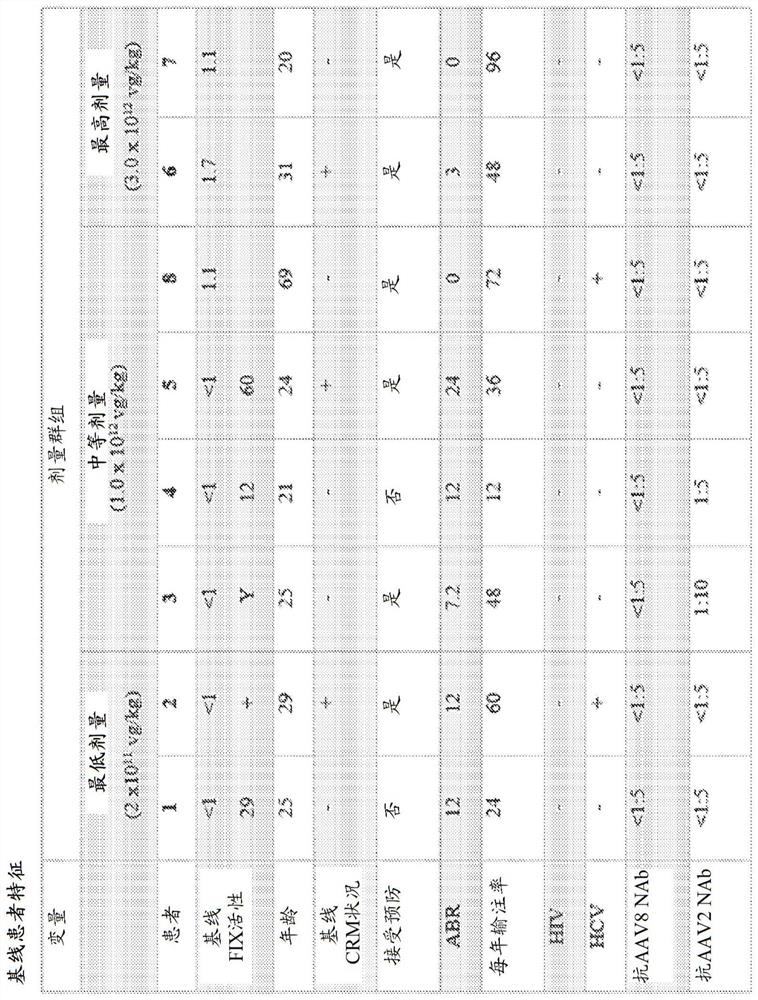
[0291] Example 1 - Whole-exome sequencing of patients treated with adeno-associated virus serotype 8-factor IX (AAV8-FIX) gene therapy reveals potential determinants of sustained transgene expression
[0292] The safety and kinetics of FIX gene therapy in patients with hemophilia B are investigated in a Phase I / II clinical trial to assess the effect of treatment on FIX activity levels and bleeding rates, and to monitor patients.
[0293] First-in-human phase I / II prospective multicenter open-label study (NCT01687608) using a non-randomized (unmixed single-arm study) single-ascending dose design to evaluate the FIX gene therapy construct FIX Padua gene therapy in patients with hemophilia Safety and kinetics in adults with disease B. The study was conducted according to the standards of Good Clinical Practice and the principles of the Declaration of Helsinki. Ethical approval was obtained from the institutional review boards of all clinical sites. The study protocol was sponso...
Embodiment 2
[0351] Example 2 - Increased immunogenicity of CpG-containing adeno-associated virus serotype 8 (AAV8) constructs may contribute to decreased transgene expression
[0352] AAV8 gene therapy has shown efficacy in clinical trials. However, an early spontaneous decline in transgene expression has been observed in some patients. It was hypothesized that an anti-AAV8-specific T cell response killed the transduced hepatocytes, resulting in a decrease in transgene expression and an increase in ALT and AST levels. To date, animal models have not shown a spontaneous decrease in transgene expression, making it difficult to analyze vector immunogenicity with respect to a decrease in transgene activity. Therefore, a murine model and a 3D bioreactor model using primary human hepatocytes were developed to assess the immunogenicity of AAV8 vectors. As a first use of the model, the impact of immune-activating CpG islands in the human FIX (huFIX) AAV8 vector (AAV8-huFIX) on the immunogenicit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com