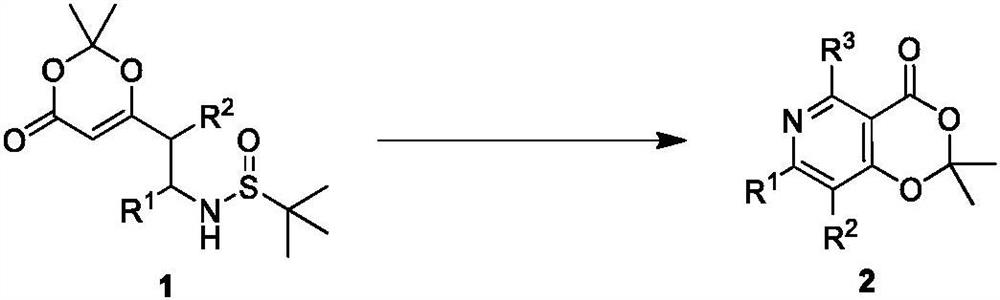
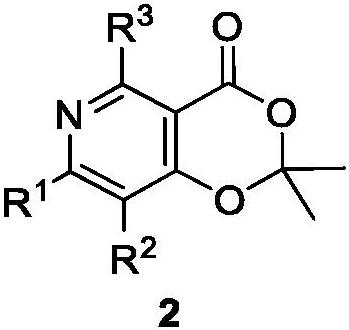
Polysubstituted pyridine derivative and preparation method thereof
A derivative and multi-substitution technology, which is applied in the field of multi-substituted pyridine derivatives and its preparation, can solve the problems of single preparation method of multi-substituted pyridine, and achieve the effects of reducing costs, avoiding heavy metal pollution, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Example 1: 7-phenyl-2,2-dimethyl-4H-[1,3]-dioxino[5,4-c]pyridin-4-one, the yield is 75%.
[0217]
[0218] 1 H NMR (400MHz, CDCl 3 )δ9.13(s,1H),8.02–8.00(m,2H),7.49–7.46(m,3H),7.30(s,1H),1.78(s,6H). 13 C NMR (100MHz, CDCl 3 ): δ164.36, 162.83, 159.67, 151.74, 137.76, 130.63, 129.02, 127.45, 108.42, 108.13, 107.68, 26.06.
Embodiment 2
[0219] Example 2: 7-phenyl-2,2-dimethyl-4-oxo-4H-[1,3]-dioxino[5,4-c]pyridine-8-carbaldehyde, yield 10 %.
[0220]
[0221] 1 H NMR (400MHz, CDCl 3 )δ9.93(s,1H),9.27(s,1H),7.61–7.57(m,2H),7.57–7.52(m,3H),1.85(s,6H). 13 C NMR (100MHz, CDCl 3 )δ188.34, 168.50, 161.96, 158.94, 153.90, 136.72, 130.96, 130.55, 128.92, 119.03, 108.73, 108.69, 26.19.
Embodiment 3
[0222] Example 3: 7-(4-chlorophenyl)-2,2-dimethyl-4H-[1,3]-dioxino[5,4-c]pyridin-4-one, yield 70 %.
[0223]
[0224] 1 H NMR (400MHz, CDCl3 )δ9.13(s,1H),7.97(d,J=8.1Hz,2H),7.46(d,J=8.1Hz,2H),7.28(s,1H),1.79(s,6H). 13 C NMR (100MHz, CDCl 3 )δ163.03, 163.00, 159.55, 151.85, 137.02, 136.17, 129.33, 128.79, 108.64, 108.07, 107.86, 26.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com