Application of fba8 gene or fba8 protein in preparation of reagents with phosphotransfer activity and/or proteolysis activity
A technology of proteolysis and protein, applied in the field of multifunctional enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
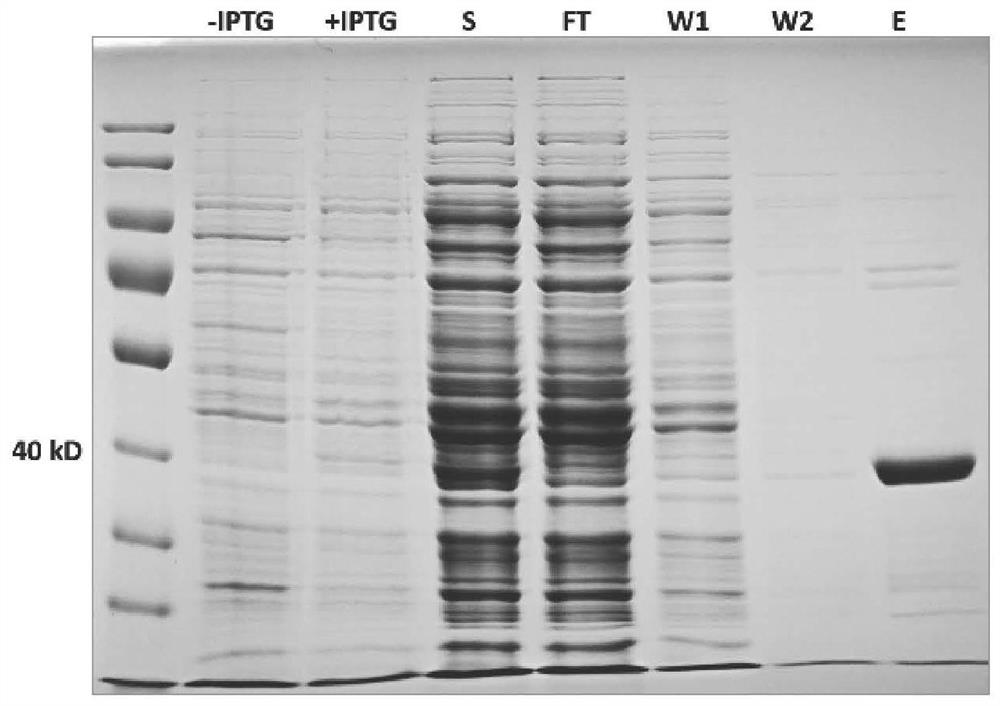
[0041] Cloning, Expression and Purification of Multifunctional Enzyme FBA8
[0042] 1. Acquisition of Arabidopsis materials
[0043] Arabidopsis thaliana were cultured in a culture chamber with a temperature of 23°C, a humidity of 50%, and a photoperiod of 16 hr light / 8 hr dark.
[0044] 2. Extraction of rachis stem RNA from Arabidopsis thaliana and reverse transcription into cDNA
[0045] When the plant height is close to 20 cm, cut off the rachis without flowers. After the material was ground with liquid nitrogen, total RNA was extracted with an RNA extraction kit (Adelai Company Plant RNA Rapid Extraction Kit RN38-EASYspinPlus). The extracted total RNA was reversely transcribed into cDNA using a reverse transcription kit (Adler's first-strand reverse transcription kit PC18-TRUEscript 1st Strand cDNASynthesis Kit).
[0046] 3. Target gene cloning
[0047] (1) Primer design
[0048] FBA8 (AT3G52930) gene sequence encodes 358 amino acids, and its CDS and amino acid sequen...
Embodiment 2
[0069] Heterologously expressed FBA8 has fructose-1,6-bisphosphate aldolase activity and its influencing factors.
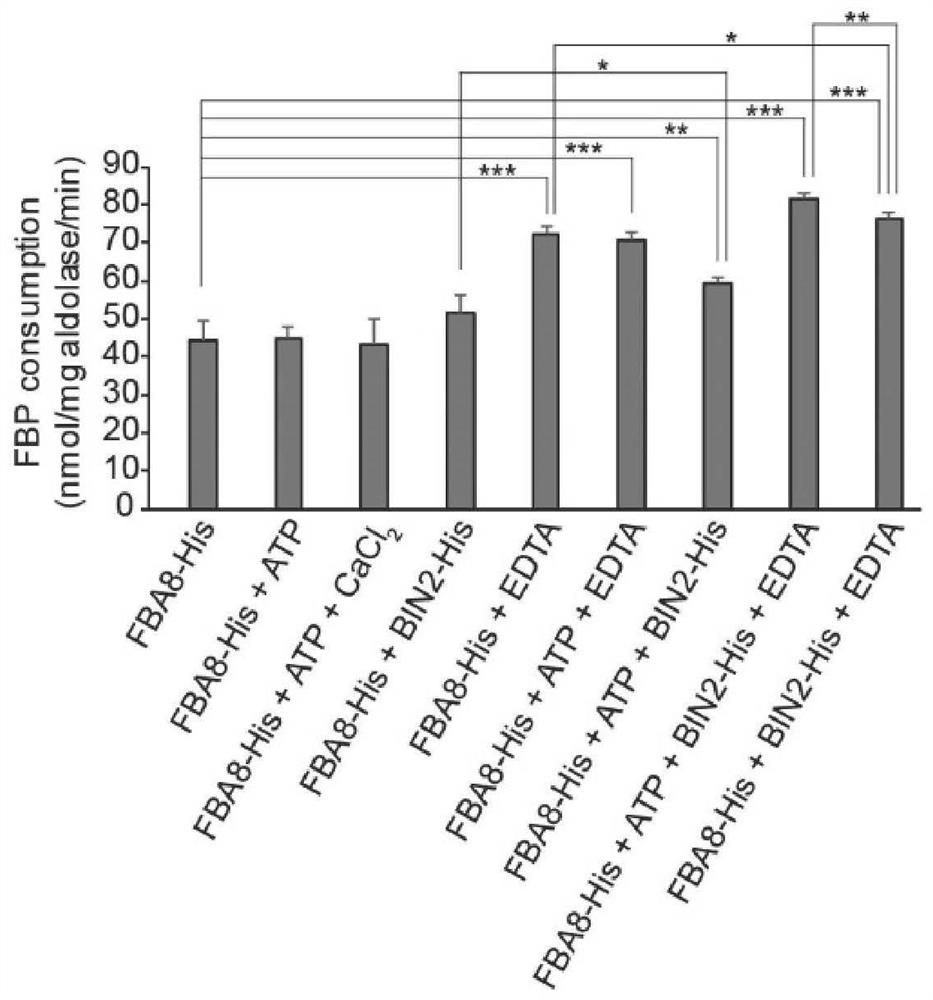
[0070] The FBA8 protein solution obtained from Example 1 was used to detect the aldolase activity with an aldolase detection kit (BC2000 from Soleibo Company). The final concentration of FBA8 was 0.18 mg / mL, and the incubation time was 1 hour. figure 2 It was shown that FBA8 with aldolase activity can be obtained by the above heterologous expression system.
[0071] Application of aldolase detection kit to detect ATP, EDTA, CaCl 2 , The effect of BIN2 protein (an interacting protein of FBA8, AT4G18710) on the activity of FBA8 aldolase. The final concentration of FBA8 is 0.18 mg / mL, the final concentration of ATP is 55.6 μM, the final concentration of EDTA is 27.8 mM, and the final concentration of CaCl 2 The final concentration of BIN2 is 0.56 mM and the final concentration of BIN2 is 0.06 mg / mL. like figure 2 As shown, EDTA can nearly double the activity o...
Embodiment 3
[0073] Heterologously expressed FBA8 has kinase activity and influencing factors.
[0074] The FBA8 protein solution (final concentration 0.24 mg / mL) obtained from Example 1 was added to the kinase reaction system (25 mM Tris-HCl, pH 7.5, 0.5 mM DTT, 10 mM MgCl 2 , 1mMATP), incubated at 37°C for 1 hour, and then commissioned Beijing Qinglian Biotech Co., Ltd. to carry out LC-MS / MS mass spectrometry detection, and it was detected that the FBA8 protein itself has many phosphorylation sites. like image 3 Shown (pep peptide containing this site is not phosphorylated; pep-Pi peptide containing this site is phosphorylated at this site; 2pep means mass spectrometry detected 2 peptides containing this site but not phosphorylated , other numbers +pep and so on; 1pep-Pi means that mass spectrometry detects 1 polypeptide containing phosphorylated at this site, other numbers +pep-Pi and so on; 2pep+1pep-Pi means mass spectrometry detects 2 peptides at the same time A polypeptide that c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com