Improved (alpha-diimine) nickel catalyst as well as preparation method and application thereof
A nickel catalyst and catalyst technology, applied in the direction of chemical instruments and methods, nickel organic compounds, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can solve the problem of the decrease of the branching degree of branched polyethylene and the inability to Ideally improve the overall performance and elastic weakening of branched polyethylene elastic materials, and achieve the effect of comprehensive performance improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 prepares ligand compound C4
[0051] In the 50mL pear-shaped bottle, add 0.17gZnCl 2, 0.2g of compound C3, 15mL of glacial acetic acid and 0.64g of 4-bromo-2,6-diisopropylaniline were stirred and refluxed at 120°C for 3h, and a solid precipitated out. Cool to room temperature, filter, wash with acetic acid three times, remove acetic acid with ether, and finally dissolve the solid with dichloromethane, add potassium oxalate to the solution, and stir for 15 minutes. After stirring, pour the solution into a separatory funnel, oscillate and let it stand, the lower organic phase liquid is released from below, the upper layer liquid is washed with water three times, then the organic phase liquid is lowered, and the upper layer waste liquid is poured out. Add MgSO to the organic phase liquid 4 Dry and stir for 15 minutes until clear. Finally, after the solution was rotary evaporated, it was placed in a ligand oven and sucked dry for one day to obtain a crude C4...
Embodiment 2
[0053] Embodiment 2 prepares catalyst Cat-B
[0054] Weigh 0.126g (DME)NiBr from the glove box 2 Put it in a 100mL pear-shaped bottle, dissolve it with 10mL of refluxing dichloromethane, then weigh 0.305g of ligand C4 and dissolve it with 10mL of dichloromethane and inject it into the pear-shaped bottle through long needle injection. The reaction was stirred at 25° C. for 24 hours, then the dichloromethane was sucked dry, the solid was washed 4 times with 25 mL of ether, and the ether was drained to obtain 0.19 g of a reddish-brown powder solid with a yield of 41.5%.
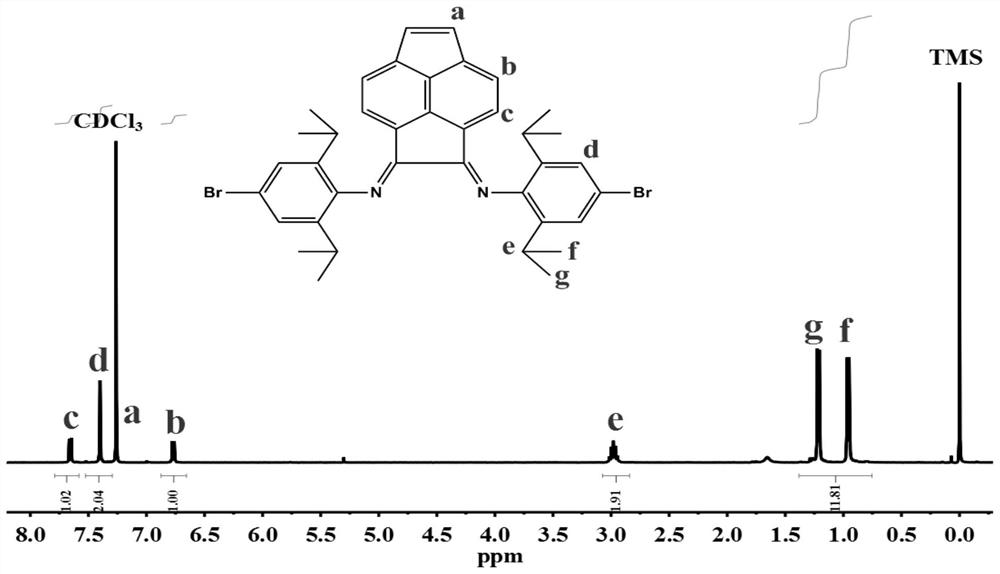
[0055] The stretching vibration absorption peak of the C=N double bond before and after complexation changes from 1629cm -1 and 1665cm -1 It becomes 1611cm -1 and 1646cm -1 , moving to the low wave number direction, indicating that a coordination effect has occurred between the N atom and the Ni atom, and the catalyst is successfully complexed.
Embodiment 3
[0056] Embodiment 3 prepares the single crystal of Cat-B
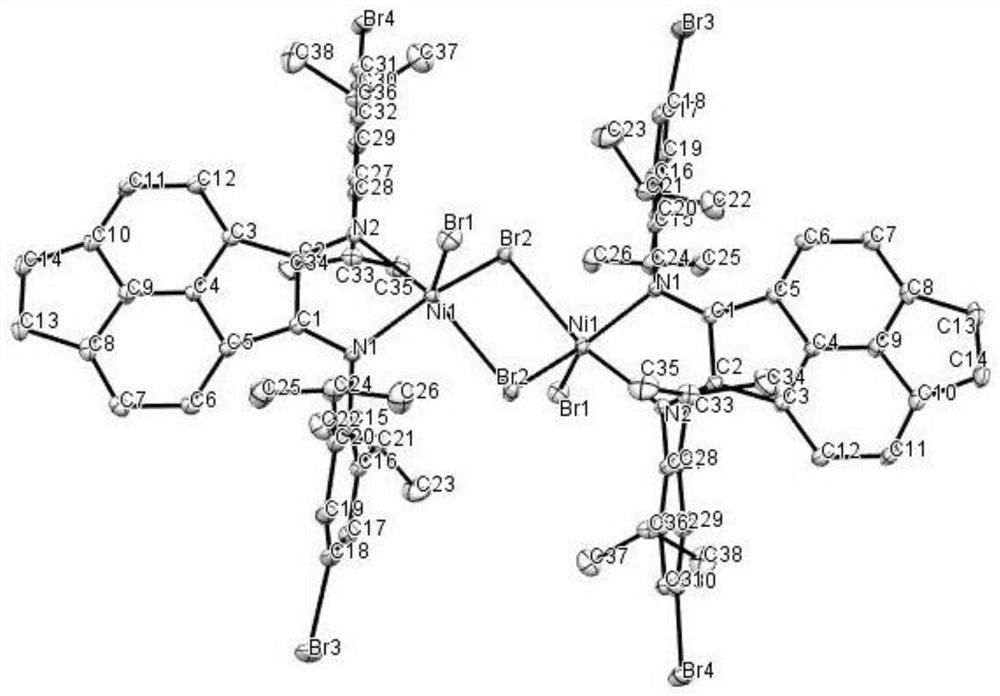
[0057] Add diethyl ether dropwise to the saturated dichloromethane solution of Cat-B, the ratio of dichloromethane to diethyl ether is about 1:5, let it stand for diffusion, and when the single crystal of Cat-B is clearly observed, select a suitable size Cat -B single crystal structure determination, the obtained measurement results are as follows figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com