HPLC detection method for L-malic acid related substances
A detection method and a technology for related substances, applied in the field of medical detection, can solve the problems of inability to effectively separate L-malic acid dimer impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Instruments and conditions:
[0029] High performance liquid chromatography: Hitachi L-2000;
[0030] Chromatographic column: C18 column (250mm×4.6mm, 3.5μm);
[0031] Column temperature: 35°C;
[0032] Mobile phase: 20mmol / L sodium dihydrogen phosphate aqueous solution, pH 2.6;
[0033] Flow rate: 0.5mL / min;
[0034] Detection wavelength: 210nm;
[0035] Injection volume: 80μL;
[0036] Elution method: isocratic elution;
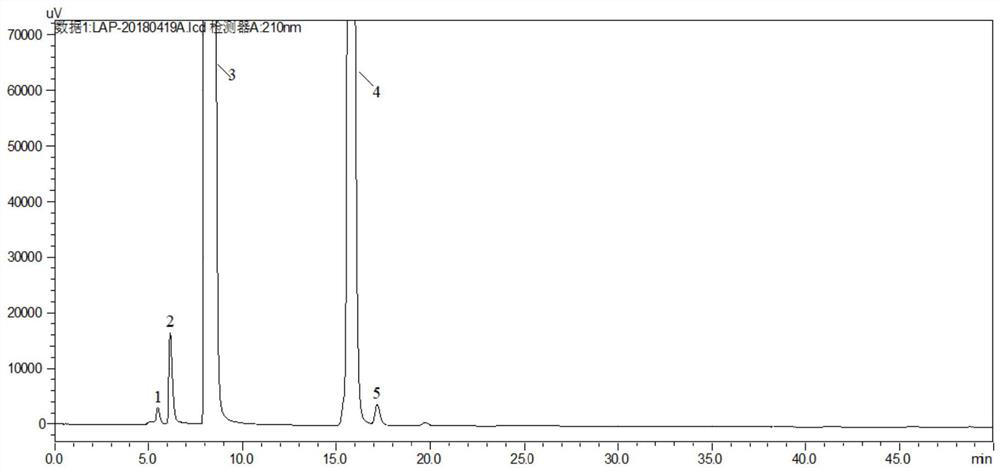
[0037] Need testing solution: take L-malic acid need testing 250mg, accurately weigh, place in 25mL measuring bottle, dilute to scale with mobile phase, as need testing solution;
[0038] The self-contrast solution of the test product: accurately measure an appropriate amount of the test product solution, dilute it 1000 times with the mobile phase, and use it as the self-control solution of the test product;
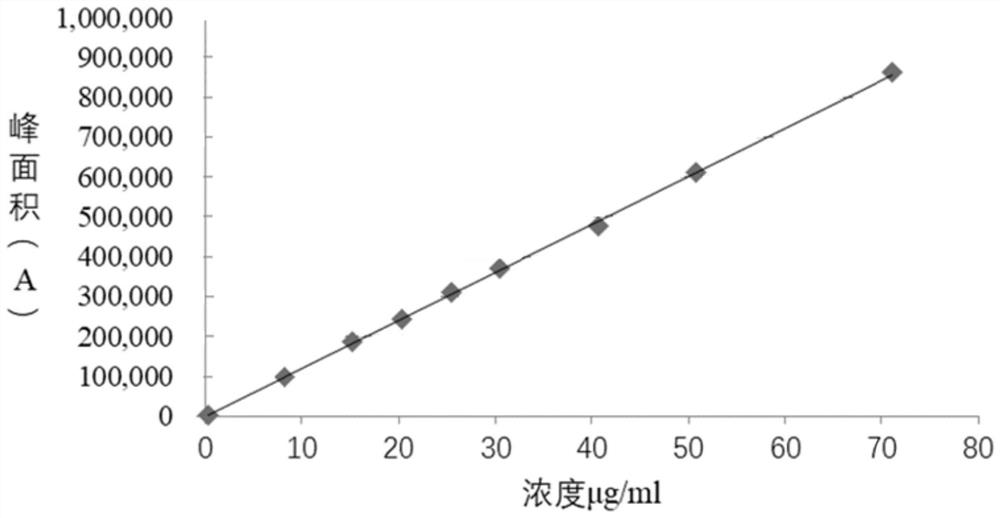
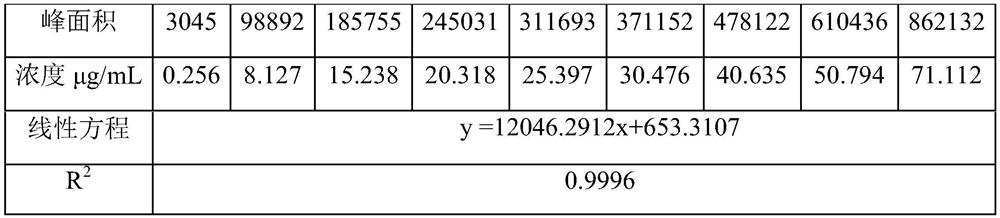
[0039] Reference substance solution: in order to ensure product quality, the present embodiment carries out the preparation of refer...
Embodiment 2
[0048] 1. Instruments and conditions:
[0049] High performance liquid chromatography: Hitachi L-2000;
[0050] Chromatographic column: C18 column (250mm×4.6mm, 3.5μm);
[0051] Column temperature: 20°C;
[0052] Mobile phase: 5mmol / L sodium dihydrogen phosphate aqueous solution, pH 2;
[0053] Flow rate: 0.1mL / min;
[0054] Detection wavelength: 210nm;
[0055] Injection volume: 1μL;
[0056] Elution method: isocratic elution;
[0057] Need testing solution, need testing self contrast solution, reference substance solution and system suitability solution are identical with embodiment 1.
[0058] 2. Detection method
[0059] The detection method is the same as in Example 1.
[0060] The results of the related substance content (fumaric acid, maleic acid, tartaric acid, L-malic acid dimer, other individual impurities and other total impurities) in the need testing solution obtained through testing are basically the same as the detection results of Example 1. quite.
Embodiment 3
[0062] 1. Instruments and conditions:
[0063] High performance liquid chromatography: Hitachi L-2000;
[0064] Chromatographic column: C18 chromatographic column (250mm×4.6mm, 5μm);
[0065] Column temperature: 40°C;
[0066]Mobile phase: 100mmol / L sodium dihydrogen phosphate aqueous solution, pH 5;
[0067] Flow rate: 1mL / min;
[0068] Detection wavelength: 210nm;
[0069] Injection volume: 100μL;
[0070] Elution method: isocratic elution;
[0071] Need testing solution, need testing self contrast solution, reference substance solution and system suitability solution are identical with embodiment 1.
[0072] 2. Detection method
[0073] The detection method is the same as in Example 1.
[0074] The results of the related substance content (fumaric acid, maleic acid, tartaric acid, L-malic acid dimer, other individual impurities and other total impurities) in the need testing solution obtained through testing are basically the same as the detection results of Example...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com