A kind of synthetic method of polyferric sulfate and polyferric sulfate
A technique of polymerizing ferric sulfate and its synthesis method, which is applied in the fields of ferric sulfate, chemical instruments and methods, flocculation/sedimentation water/sewage treatment, etc., and can solve the problems that the wastewater treatment effect needs to be further improved and the impurity adsorption effect is not good. Improve the ability to remove impurities, improve the effect of water purification, and improve the effect of lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1, a polymeric sulfate, synthesized by steps.
[0048] S1, taking seven hydrated sulfate 55.6 g (0.2 mol), dissolved in 30 ml of water, slowly added 2 ml of mass fraction of 40% sulfuric acid, heated to 50 °, stirred to uniform, to obtain a first mixing solution;
[0049] S2, the first mixed solution continues to 70 ° C, 0.4 g of sodium nitrite is added disposable, and the air is transferred, and the stirring reaction is held for 150 min to obtain a second mixed solution;
[0050] S3, the second mixed solution is cooled to 55 ° C, and the organic skeleton and a co-solvent is added to the second mixed solution, wherein the organic skeleton is a terephthalic acid, an addition amount of 3.32 g (0.02 mol), the co-solvent is ethanol, help The solvent was added 10 mL, and after the organic skeleton and a co-solvent were added, stirring was continued and reacted for 3 h, resulting in a third mixed solution;
[0051] S4, post-treatment of the third mixed solution, specifica...
Embodiment 2~14
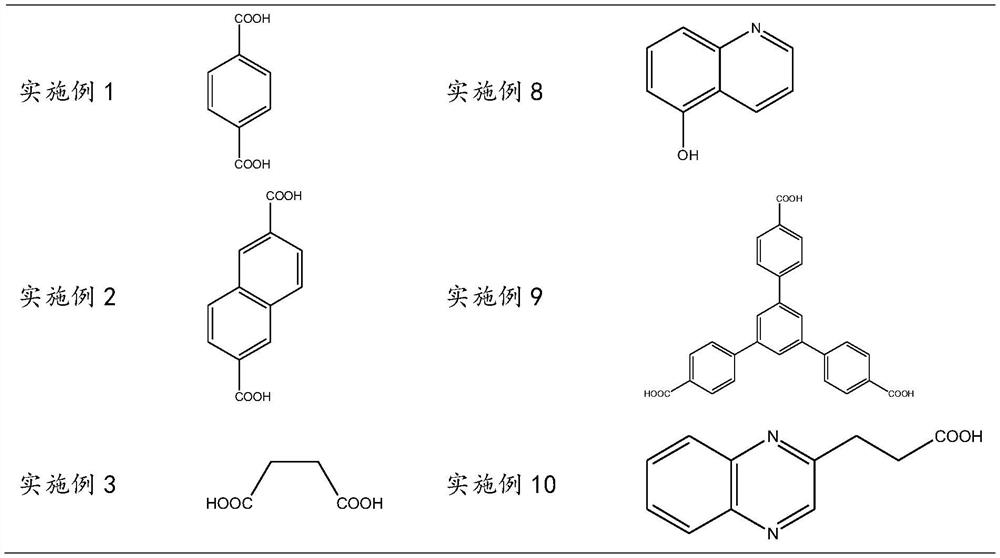
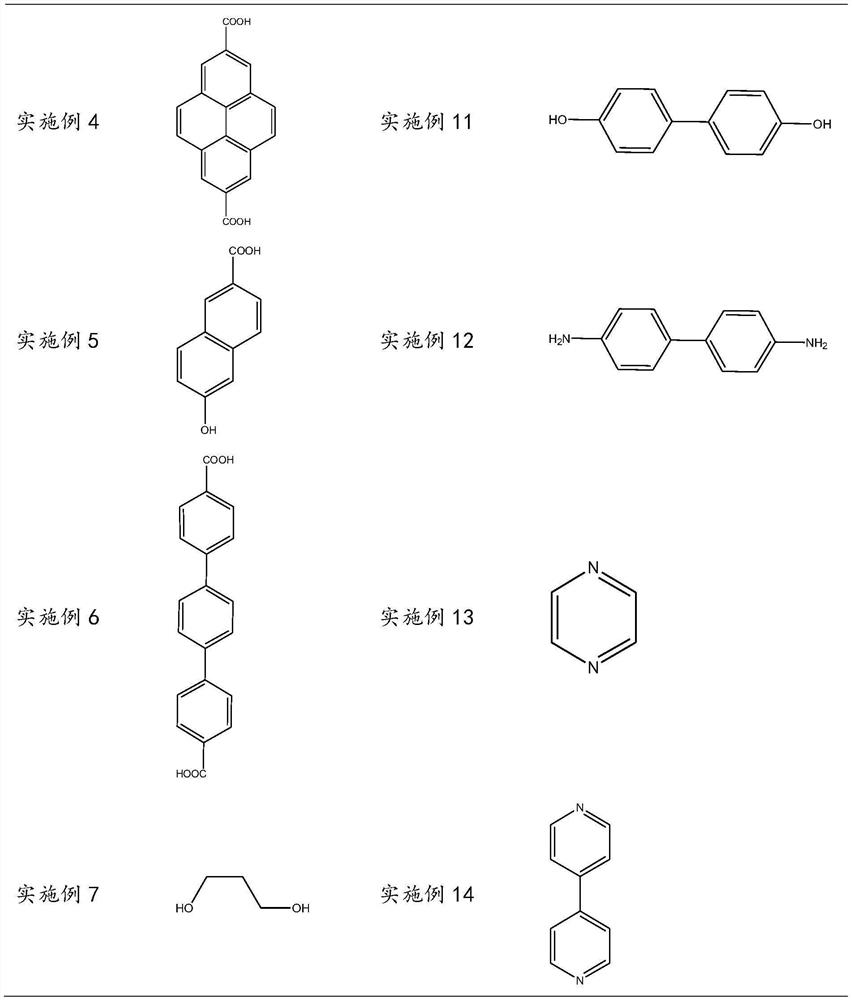
[0055] Examples 2 to 14, a polymeric sulfate, distinguishing between Example 1 is that the organic skeleton is replaced with the substance shown in Table 1 at an amount of the same substance.
[0056] At the same time, the comparison is selected as follows.
Embodiment 15~20
[0067] Examples 15 to 20, a polymeric sulfate, the difference from Example 1 is that the organic skeleton is replaced with the same substance to be as shown in Table 3.
[0068] Table 3, the structure of the organic skeleton in Examples 15 to 20
[0069]
[0070]
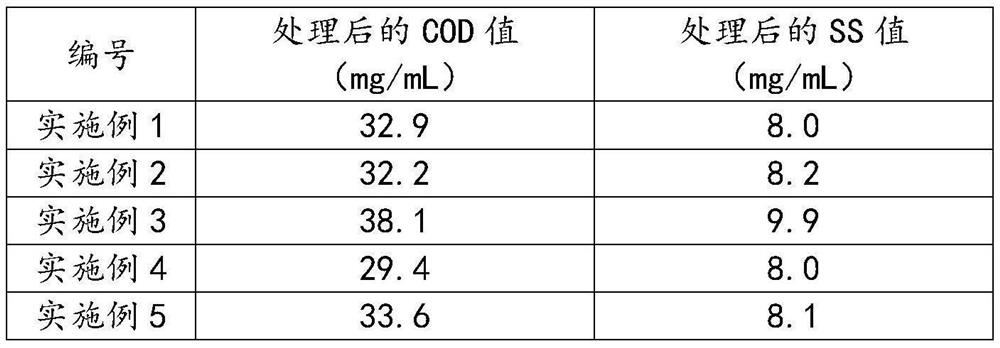
[0071] The polymerized sulfate prepared in the above embodiment was prepared, and the sewage treatment experiment was carried out, and the results were shown in Table 4.
[0072] Table 4, the sewage treatment results of Examples 15 to 20
[0073] serial number COD value (Mg / ml) after processing SS value (mg / ml) after treatment Example 15 29.6 7.5 Example 16 29.2 7.4 Example 17 27.7 6.9 Example 18 27.1 7.0 Example 19 34.0 8.1 Example 20 27.2 7.0
[0074] In Examples 15 to 20, the alkoxy group or alkyl group is connected to the benzene ring chain can be carried out by a Fouroyylation reaction, a Four alkylation reaction, a halogenated suzuki reaction, and the like...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com