Nucleic acid composition, kit and method for detecting pathogens of upper respiratory diseases of cats
A nucleic acid composition and a technology for the upper respiratory tract, applied in the field of molecular biotechnology in vitro diagnosis, can solve the problems of long detection period, low sensitivity, single detection pathogen, etc., and achieve the effects of high sensitivity, good detection specificity, and rapid and accurate detection method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] This embodiment provides a nucleic acid composition for detecting pathogens of feline upper respiratory tract diseases.
[0112] The nucleic acid composition provided in this embodiment includes: a first nucleic acid combination for detecting feline herpes type 1 virus, a second nucleic acid combination for detecting feline calicivirus, a third nucleic acid combination for detecting feline mycoplasma, and a combination for detecting feline calicivirus. A fourth nucleic acid combination for detecting Chlamydia felis and a fifth nucleic acid combination for detecting Bordetella bronchiseptica;
[0113] The first nucleic acid combination comprises the first primer pair and the first probe, the second nucleic acid combination comprises the second primer pair and the second probe, the third nucleic acid combination comprises the third primer pair and the third probe, and the fourth nucleic acid combination comprises The fourth primer pair and the fourth probe, the fifth nucl...
experiment example 1
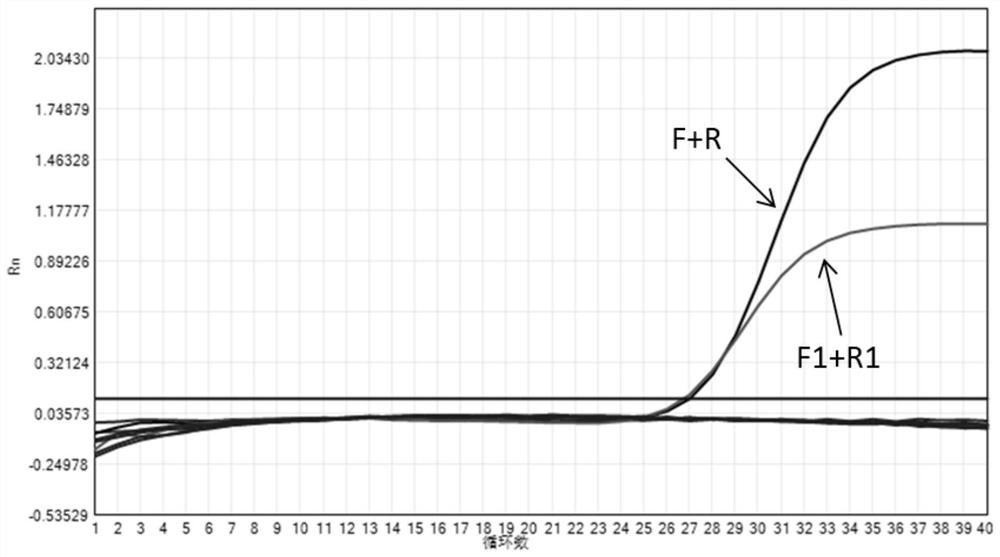
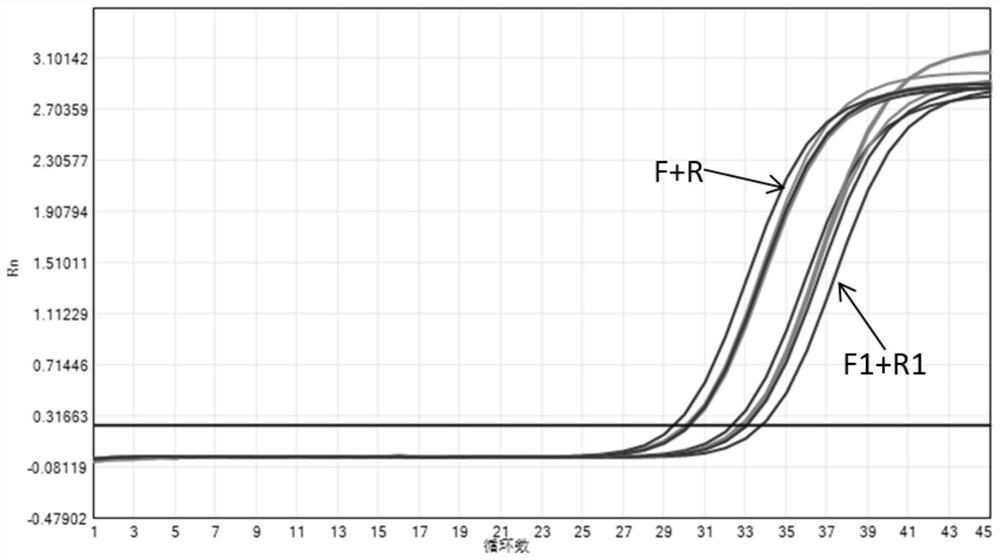
[0139] In this experimental example, the nucleic acid composition provided in Table 1 of Example 1 and the nucleic acid composition provided in Comparative Example 1 were used to detect pathogens of upper respiratory tract diseases in cats respectively. The primers in Table 1 and Table 2 were synthesized by Thermo Fisher Scientific.
[0140] Processing of samples to be tested: Extract nucleic acid from clinical samples of feline herpes virus type 1, feline calicivirus, feline mycoplasma, feline chlamydia, and bronchiseptica Bordetella respectively, and the extracted pathogenic nucleic acid is first used in the commercially available PCR of Y manufacturer Individual detection reagents are verified to confirm that each pathogen is positive. In this experimental example, the samples to be tested for feline herpes virus type 1, feline mycoplasma, feline chlamydia and Bordetella bronchiseptica are DNA samples, and the test samples for feline calicivirus are RNA samples.
[0141] U...
experiment example 2
[0148] Perform primer specificity experiments. The positive pathogen samples in Experimental Example 1 were amplified using each set of primers provided in Example 1, and the amplified products were entrusted to Xi'an Qingke Biotechnology Co., Ltd. for electrophoresis and sequencing.
[0149] Amplification program: 50°C, reverse transcription for 10 minutes (the reverse transcription step is only for feline calicivirus, other pathogens do not need the reverse transcription step, and directly proceed to the subsequent pre-denaturation reaction procedure); 95°C, pre-denaturation for 3 minutes; 95°C, denaturation 5s, 60°C, annealing and extension for a total of 20s, 40 cycles.
[0150] Compared with Experimental Example 1, the reaction system differs only in that no fluorescent probe is added.
[0151] The electrophoresis pattern shows that the size of the band amplified by each primer set is consistent with the expected target fragment size, refer to Figure 16 shown.
[0152...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com