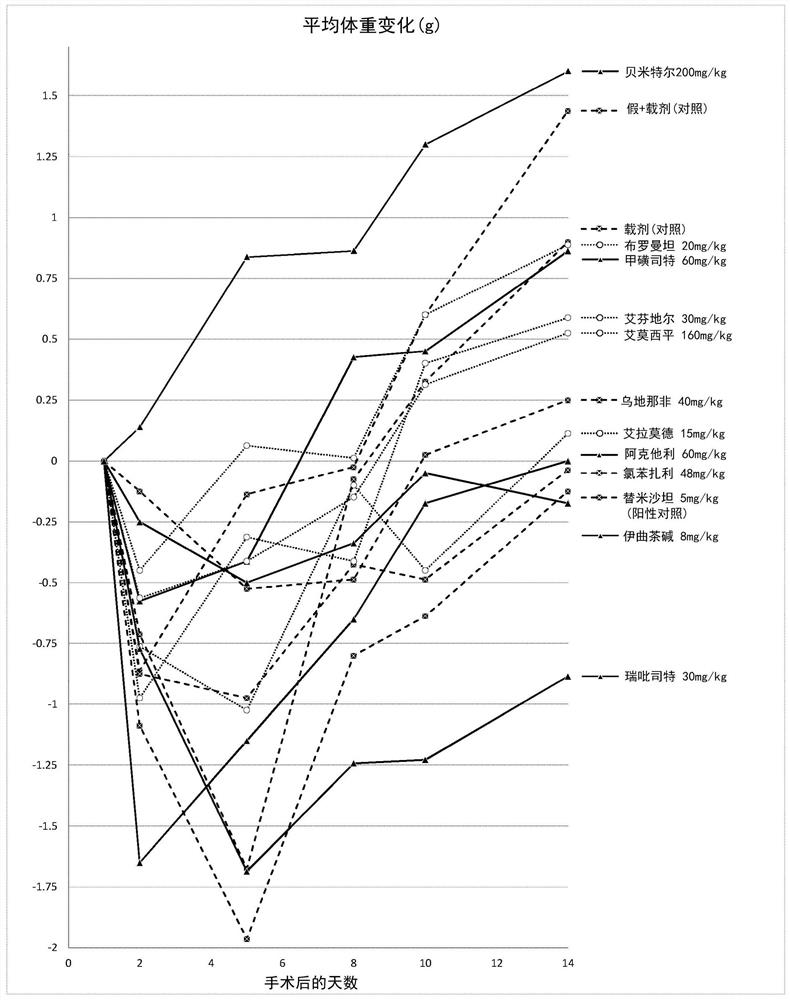
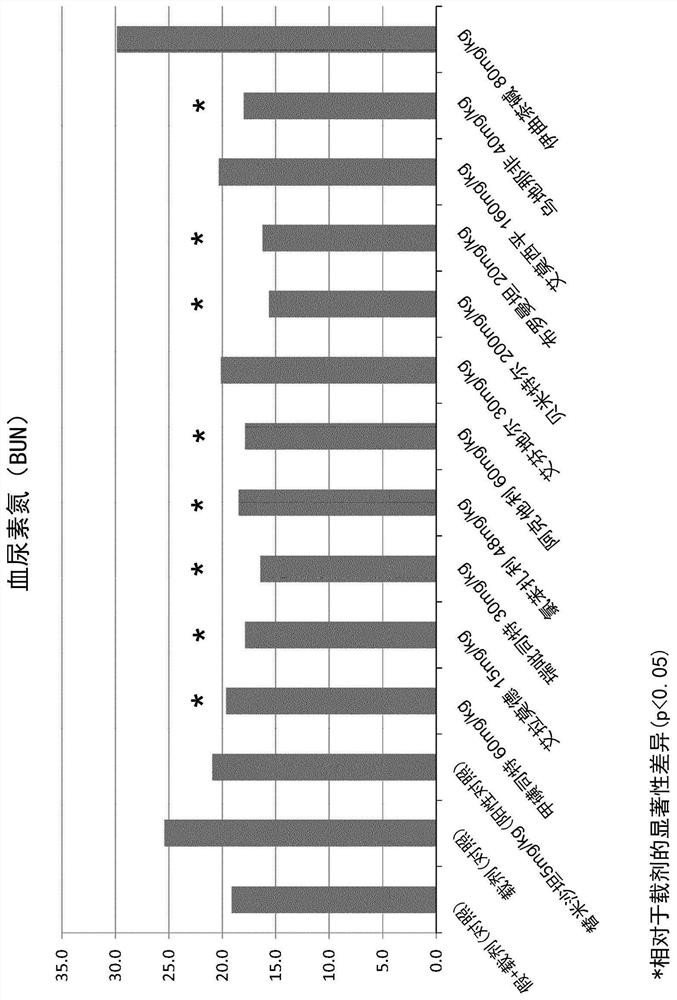
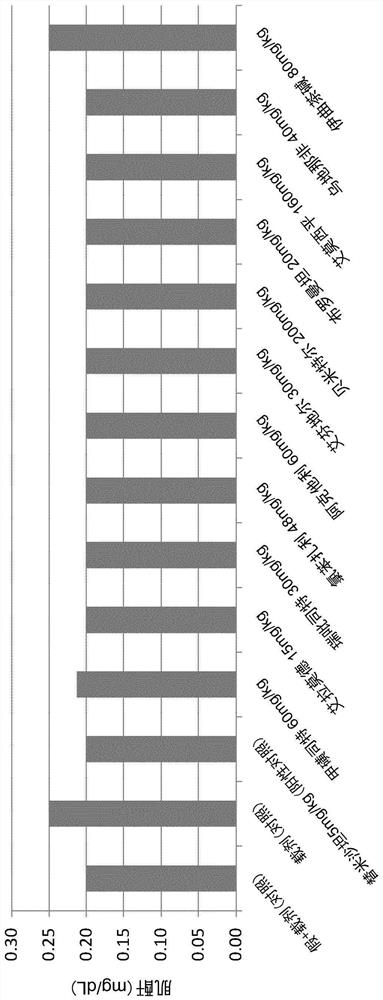
The use of actarit in the prophylaxis or treatment of renal fibrosis or kidney disease
A technology for renal fibrosis and kidney disease, applied in the direction of urinary system diseases, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., can solve problems such as renal fibrosis or CKD that cannot be cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Materials and Methods
[0120] Male 9-12 week old C57BL / 6 mice weighing 23-25 g were used. All mice were acclimatized for a minimum of 5 days prior to study initiation and were housed individually in microisolators on a 12:12 light-dark cycle throughout the study with standard mouse maintenance chow (Harlan Teklad 2018), chow and Water was obtained ad libitum. Before surgery, all mice were weighed as baseline body weight.
[0121] UUO surgical intervention was performed according to previously described methods known in the art (Le Meur Y et al., Macrophage accumulation at a site of renal inflammation is dependent on the M-CSF / c-fms pathway (Macrophage accumulation at a site of renal inflammation is dependent on the M-CSF / c-fms pathway), J Leukocyte Biol., 72:530-537; 2002).
[0122]All mice were first anesthetized with a rodent mixture (ketamine 10 mg / mL and Xyaline 1 mg / mL) in saline (10 μL / g body weight) by intraperitoneal injection. Pedal reflexes and vibris...
Embodiment 2
[0170] Materials and Methods
[0171] In this example, healthy female young C57BL / 6 mice were used for the study. Mice were between 9-12 weeks of age and weighed 23-25 g at the start of the study. All mice were obtained from Charles River Laboratories.
[0172] Mice were maintained in a controlled environment with a temperature of 70-72°F and a humidity of 30-70%, with a light cycle of 12 hours light and 12 hours dark. Harlan Teklad 2018 standard mouse maintenance chow was provided and water was available ad libitum.
[0173] After five days of acclimatization, mice were grouped according to body weight. Ten groups in total, ten mice in each group. Nine of the ten groups underwent UUO individually, while another of the ten groups underwent sham surgery as a non-operative control, as described above.
[0174] After surgery, the mice were divided into 10 separate study groups of 10 animals and received oral treatment once a day for 14 days after postoperative recovery. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com