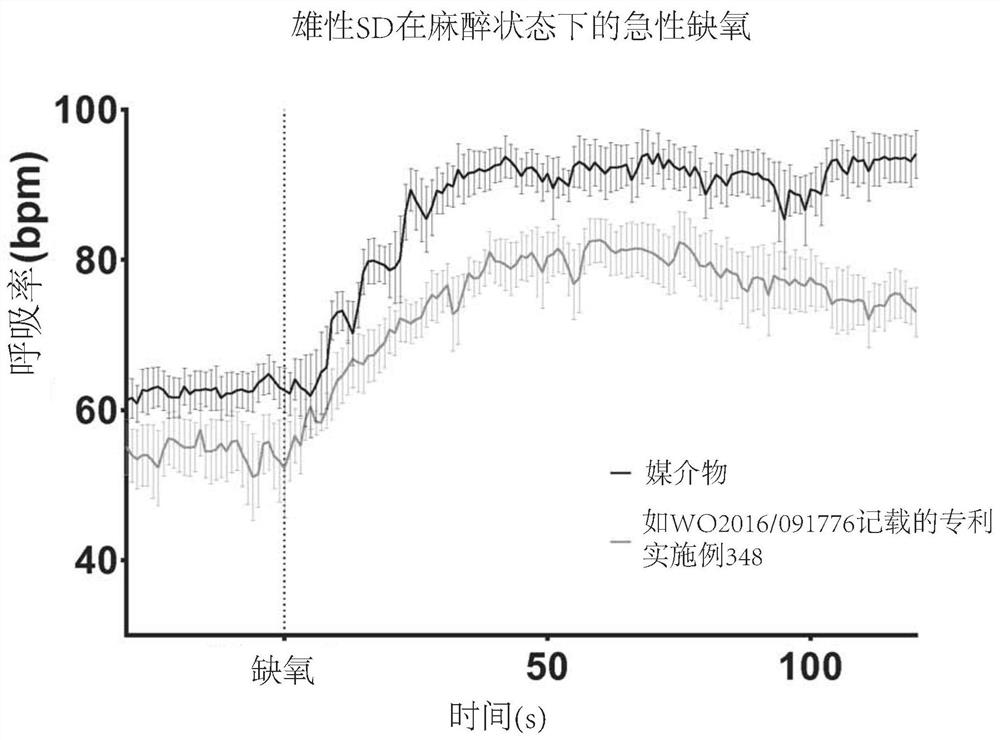
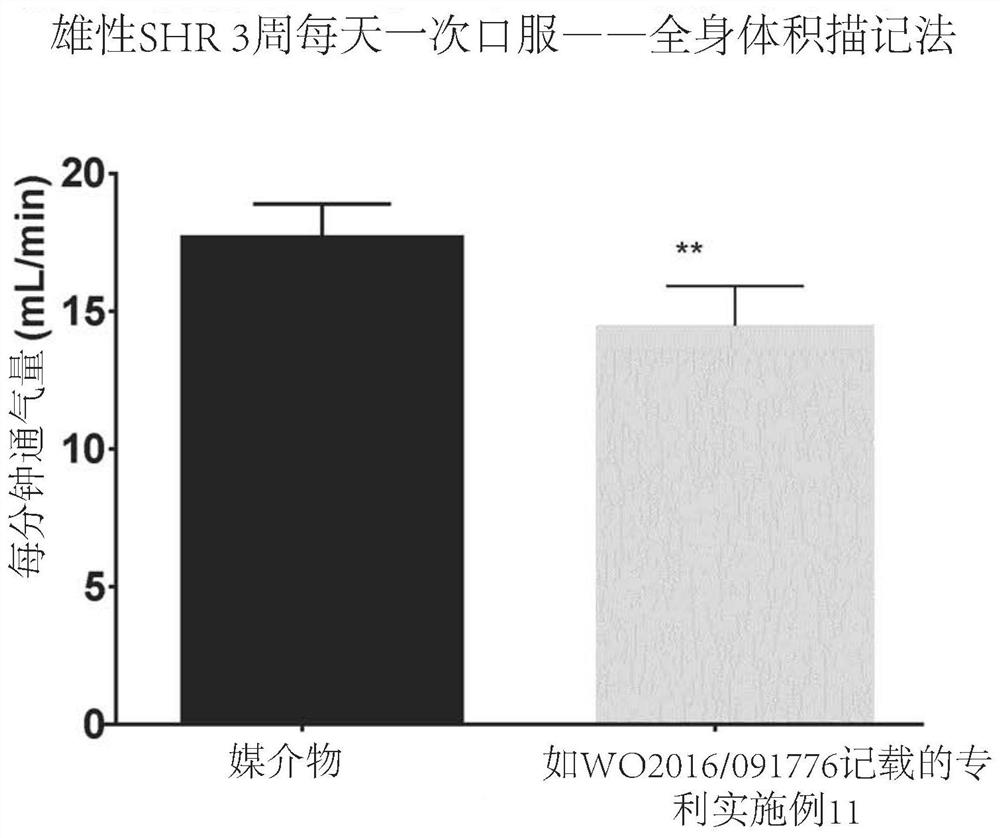
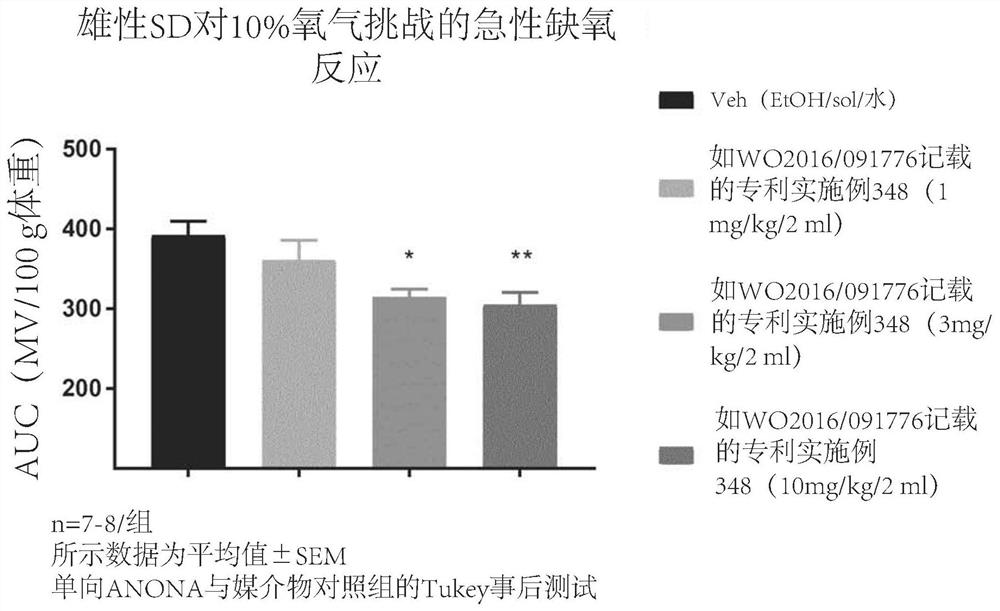
1,3-thiazol-2-yl substituted benzamides for the treatment of diseases associated with nerve fiber sensitization
An alkyl-based, multi-purpose technology, applied in the direction of nervous system diseases, respiratory system diseases, cardiovascular system diseases, etc., can solve the problems of inability to chemically reflect hypersensitivity and abnormal taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0058] The terms mentioned herein preferably have the following meanings:
[0059] The terms "halogen atom", "halo-" or "halo-" are understood to mean fluorine, chlorine, bromine or iodine atoms, preferably fluorine or chlorine atoms.
[0060] The term "alkyl" is understood to mean a linear or branched saturated monovalent hydrocarbon radical having the indicated number of carbon atoms, usually in the R 2 2 to 6 carbon atoms in the case of , and 1 to 4, preferably 1-3 carbon atoms in the case of all other alkyl substituents, for example and preferably methyl, ethyl, propyl, butyl, pentyl, Hexyl, isopropyl, isobutyl, sec-butyl, tert-butyl, isopentyl, 2-methylbutyl, 1-methylbutyl, 1-ethylpropyl, 1,2-dimethyl Propyl, neopentyl, 1,1-dimethylpropyl, 4-methylpentyl, 3-methylpentyl, 2-methylpentyl, 1-methylpentyl, 2-ethylbutyl base, 1-ethylbutyl, 3,3-dimethylbutyl, 2,2-dimethylbutyl, 1,1-dimethylbutyl, 2,3-dimethylbutyl, 1 ,3-Dimethylbutyl or 1,2-dimethylbutyl or isomers thereof. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com