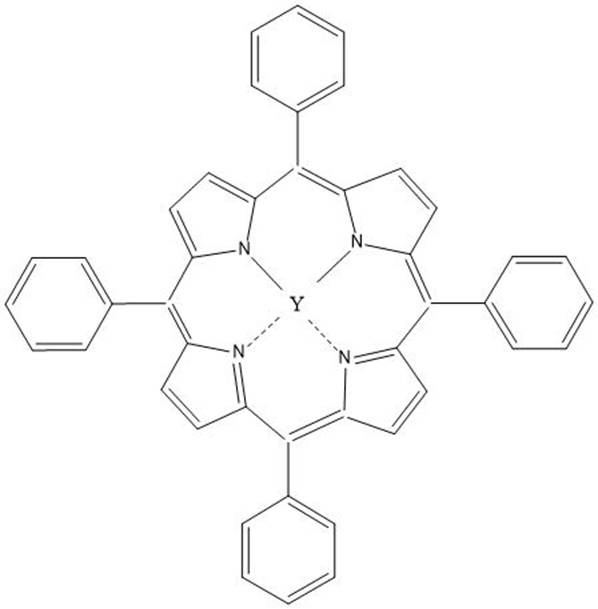
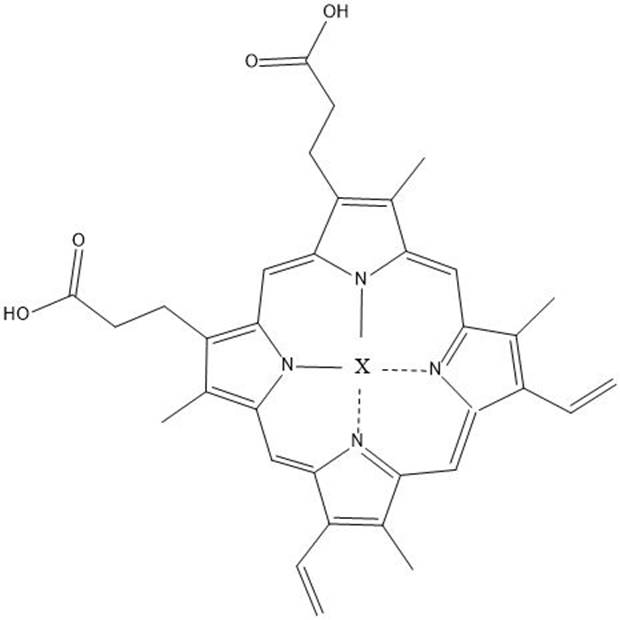
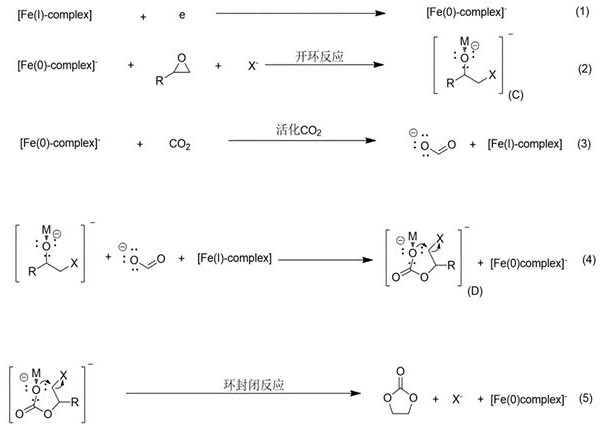
Method for electrochemical synthesis of cyclic carbonate and application of method
A cyclic carbonate, electrochemical technology, applied in the electrolysis process, electrolysis components, electrolysis organic production and other directions, can solve the problem of harsh reaction conditions and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of cyclic carbonate, comprises the following steps:
[0025] The method uses a graphite rod as a working electrode, a Pt ring as an auxiliary electrode, a silver wire as a reference electrode, tetrabutylammonium perchlorate as a supporting electrolyte, and dimethylformamide as an electrolyte. The second catalyst is obtained by catalyzing the electrochemical addition of carbon dioxide and alkylene oxide. The reaction is carried out at normal temperature and pressure. The obtained product is extracted three times by adding water and dimethyl ether, and then evaporated to obtain a cyclic carbonate;
[0026] Preparation of reference electrode: Weigh 0.0169g of nitrate (AgNO3) and dissolve it in 10mL of dimethylamide (DMF), stir to dissolve it completely, and then transfer it to the reference electrode fixed with silver wire, soak the electrode in Stay overnight in the solution configured above to stabilize the reference el...
Embodiment 1
[0064] Product preparation: Weigh 1g of tetrabutylammonium perchlorate (TBAP) and dissolve it in dimethylformamide (DMF), transfer it to a 50mL volumetric flask and constant volume, add the above electrolyte solution into the H-type electrolytic cell with a pipette gun There are two chambers, Yin and Yang, and the volume of each chamber is 10mL. The working electrode and reference electrode are placed in the cathode chamber, and the auxiliary electrode is placed in the anode chamber. High-purity nitrogen gas is introduced into the two chambers of the electrolytic cell for 15 minutes to remove dissolved oxygen. Add 50mg of complex 1 and 50mg of complex 3 to the cathode chamber of the H-type electrolytic cell, respectively, and epichlorohydrin with a molar ratio of 1:5000 to the first catalyst, feed 0.1MPa carbon dioxide for 20min, and set the electrolysis voltage -2V, Electrolysis time 2h.
[0065] Product extraction: transfer the above electrolysis product into a separatory fu...
Embodiment 2
[0070] Product preparation: Weigh 2g of tetrabutylammonium perchlorate (TBAP) and dissolve it in dimethylformamide (DMF), transfer it to a 50mL volumetric flask and constant volume, add the above electrolyte solution into the H-type electrolytic cell with a pipette gun There are two chambers, Yin and Yang, and the volume of each chamber is 10mL. The working electrode and reference electrode are placed in the cathode chamber, and the auxiliary electrode is placed in the anode chamber. High-purity nitrogen gas is introduced into the two chambers of the electrolytic cell for 15 minutes to remove dissolved oxygen. Add 100mg of complex 1 and 100mg of complex 4 to the cathode chamber of the H-type electrolytic cell, and epoxycyclopentane with a molar ratio of 1:50000 to the first catalyst, feed 8MPa carbon dioxide for 20min, and set the electrolysis voltage to -2.5V , Electrolysis time 2h.
[0071] Product extraction: transfer the above electrolysis product into a separatory funnel,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com