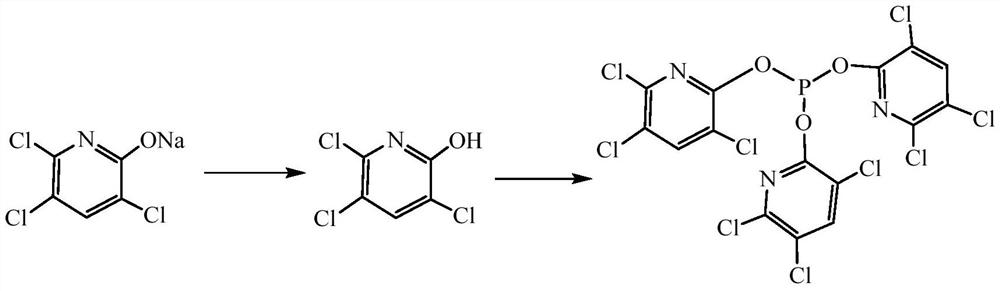
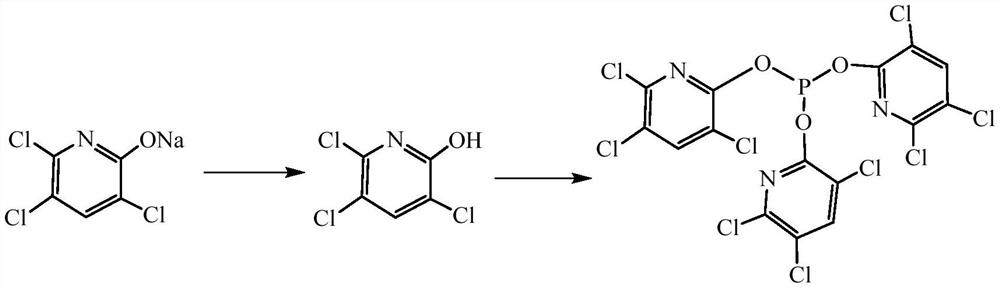
Synthesis method of tri(3,5,6-trichloropyridinyl)phosphite
A technology of trichloropyridine and a synthesis method, which is applied in the synthesis field of phosphite tri-ester, can solve the problems of increasing cost, difficulty of wastewater treatment, increasing the amount of phosphorus trichloride, low purity of target product, etc., and achieves the reduction of diester. Generation, reduction of side reactant generation, effect of mild reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Synthesis of 2-hydroxyl-3,5,6-trichloropyridine: add 100g of 85% 3,5,6- Sodium triclopyridine 2-alcohol, 280g tap water, in the process of stirring, the temperature is raised to 60°C, and the mass concentration that has been placed in the dropping tube prepared in advance is added dropwise and is 31% hydrochloric acid. Control the reaction temperature to 60°C-70°C, and adjust the pH value to 2-3. Stabilize for 10 minutes without changing the pH value, then lower the temperature to 20°C, filter with suction, and dry until the water content is ≦8%, to obtain 84.5 g of off-white solid 2-hydroxy-3,5,6-trichloropyridine, with a yield of 97.75%. Content 88.5%.
[0041] (2) Dehydration of 2-hydroxyl-3,5,6-trichloropyridine: In a 500ml four-neck flask equipped with a stirrer, a thermometer, and a glass oil-water separator, add 2-hydroxyl-3 with a content of 88.5%, Add 67.3g of 5,6-trichloropyridine, add 120g of toluene, heat up to 105°C-115°C during the stirring process, ...
Embodiment 2
[0044] In step (2), the dehydrating agent toluene is replaced by ethyl acetate, and the others are the same as in Example 1, and the ethyl acetate is distilled off under reduced pressure to obtain 61.4 g of tris-(3,5,6-trichloropyridine) phosphite , the yield was 94.83%, and the purity was 96.3%.
Embodiment 3
[0046] In step (3), the catalyst benzyltriethylammonium chloride is replaced by 4-dimethylaminopyridine, and others are the same as in Example 1, and toluene is distilled out under reduced pressure to obtain tri-(3,5,6- Triclopyridine) ester 61.8g, the yield is 96.44%, and the purity is 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com