A kind of 4-methyl-4-phenylcyclopentenone compound and preparation method thereof
A technology of phenylcyclopentenone and compound, which is applied in the field of 4-methyl-4-phenylcyclopentenone compound and its preparation, can solve problems such as activation of transition metals, and achieve mild reaction conditions and good substrate Applicability and functional group tolerance, effect of simple process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Under an inert gas atmosphere, add tetrahydrofuran (1.0ml, concentration 5mol / L) of acetaldehyde into a low-temperature reactor at -30°C, and add a tetrahydrofuran solution of ethynylmagnesium bromide dropwise under stirring (12ml , concentration 0.5mol / L, titration rate 1.0ml / min); after the reaction finishes, add excess saturated ammonium chloride solution to quench, and extract with ethyl acetate;
[0032] (2) Drying the extract, vacuum distillation, and silica gel column separation in sequence to obtain methyl acetylenone.
Embodiment 2
[0034] (1) Add acetophenone (the amount of acetophenone substance 10mmol, quality 1201.5mg, volume 1.166.5ml) to the methanol solution (20ml) of p-toluenesulfonyl hydrazide at one time, and react at 60°C under stirring conditions to A large amount of white solid appeared in the solution; the solid precipitate was washed with petroleum ether to obtain p-toluenesulfonyl hydrazone.
Embodiment 3
[0036] (1) Under argon atmosphere and stirring conditions, mix 0.2mmol methyl acetylenone, 0.4mmol p-toluenesulfonylhydrazone, 0.02mmol copper hydroxide and 1.0mmol lithium methoxide, add 4mL solvent toluene and 3.6 μL of water to obtain a reaction solution, and raise the temperature to 100°C for 12 to 24 hours;
[0037] (2) After the reaction is finished, remove insoluble solids by filtration, use a rotary evaporator for vacuum distillation, pressure 150hPa, water bath temperature 40°C, evaporation time 18-22min; use chromatography column chromatography separation technology for concentration and separation, silica gel 200-300 mesh , Developing agent polar ethyl acetate / petroleum ether=1 / 49 (v / v), to obtain the target product 4,4-disubstituted cyclopentenone.
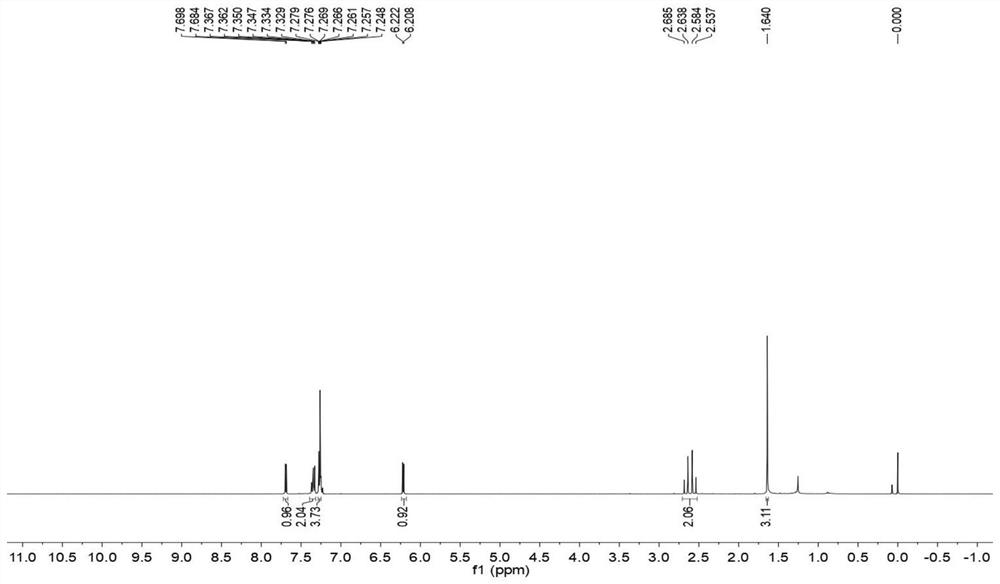
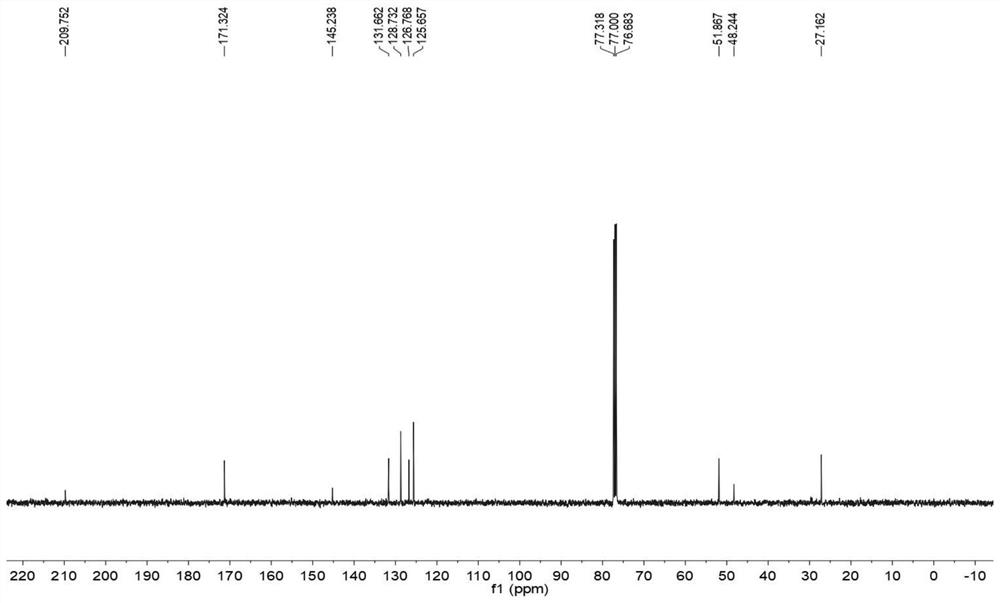
[0038] The obtained target product was detected, and the results were as follows: figure 1 , 2 as shown, figure 1 for 4-methyl-4-phenylcyclopentenone 1 H NMR spectrum, figure 2 for 4-methyl-4-phenylcyclopentenone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com