Detection method for mitiglinide calcium R-isomer
A technology of mitiglinide calcium and isomers, which is applied in the identification field of mitiglinide calcium R-isomers, can solve the problem that the resolution cannot meet legal requirements and the like, and achieves excellent detection effect, good stability, No trailing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
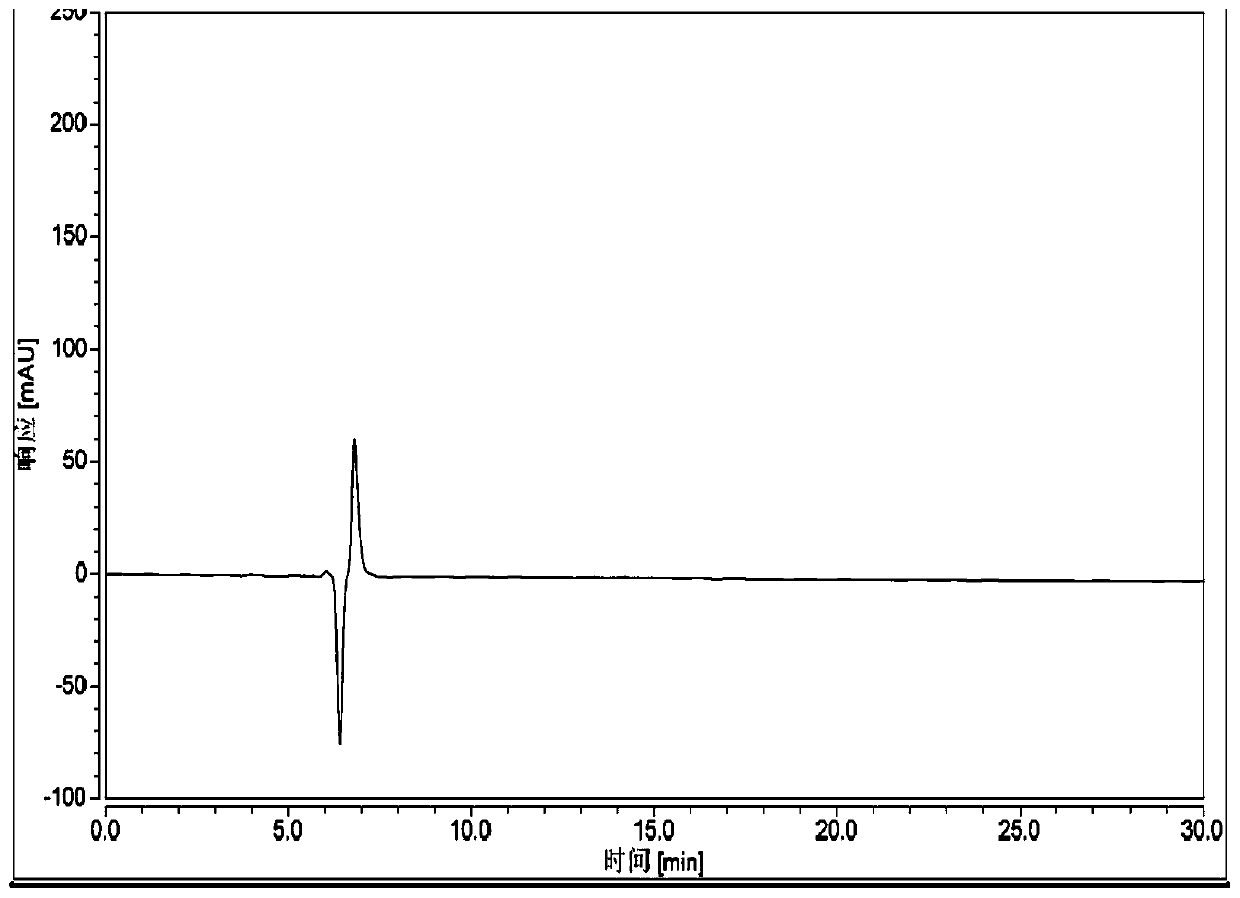
[0073] Chromatographic conditions and system suitability test:
[0074] Chromatographic column: chiral chromatographic column Ultimate Amy-DR (250×4.6mm 5μm);
[0075] Mobile phase: acetonitrile-trifluoroacetic acid (100:0.1);
[0076] Detection wavelength: 210nm.
[0077] Flow rate: 0.3ml / min
[0078] Column temperature: 30°C
[0079] Preparation of the test solution: Take an appropriate amount of the test product, weigh it accurately, add mobile phase to make a solution containing 0.2 mg per 1 ml, and shake well to obtain.
[0080] Preparation of mitiglinide calcium reference substance solution: Take an appropriate amount of the reference substance, weigh it accurately, add mobile phase to make a solution containing 0.2mg per 1ml, and shake well.
[0081] Preparation of R-isomer reference substance solution: Take an appropriate amount of R-isomer reference substance, weigh it accurately, add mobile phase to make a solution containing 0.2mg per 1ml, and shake well.
[00...
example 2
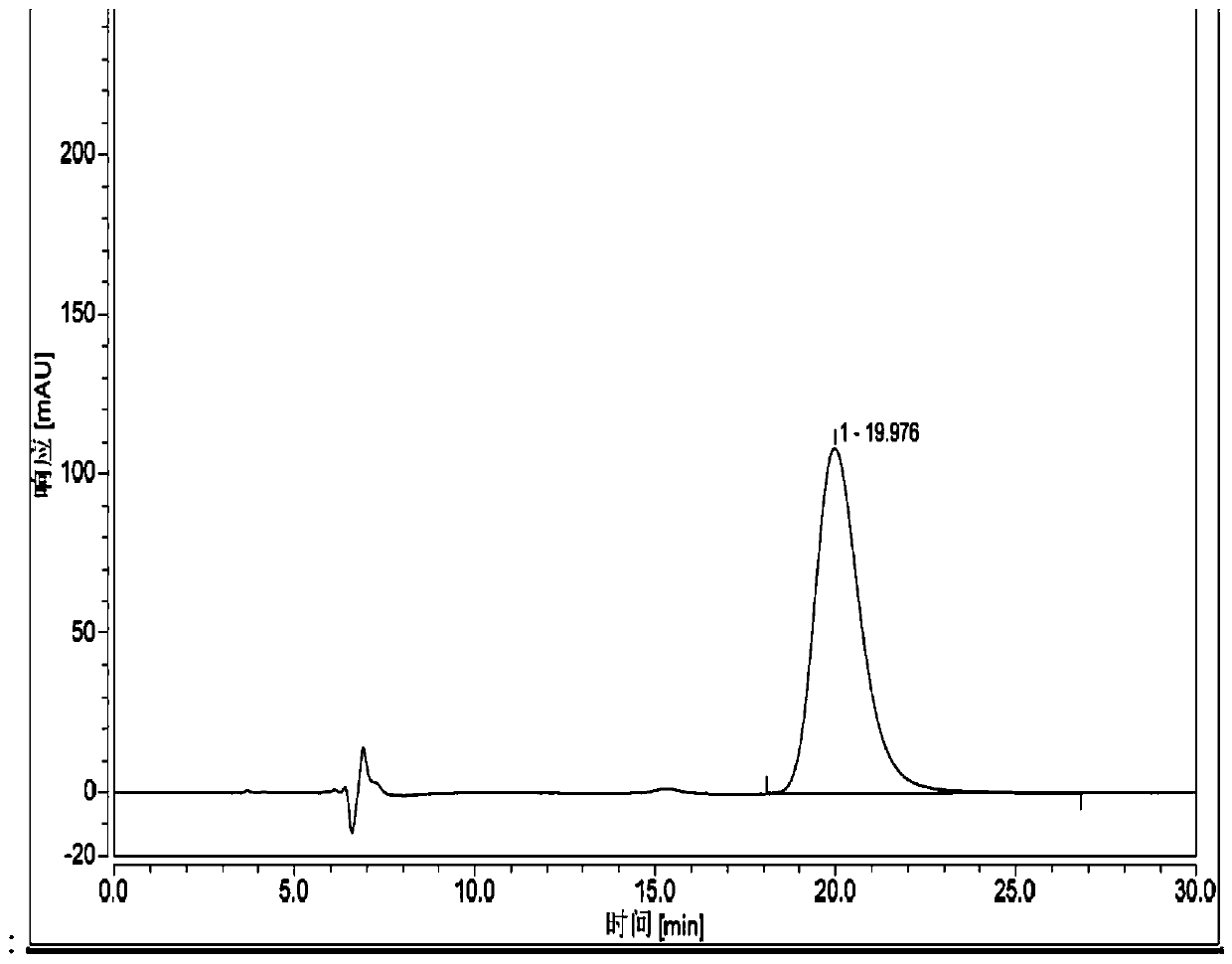
[0085] Chromatographic conditions and system suitability test:
[0086] Chromatographic column: chiral chromatographic column Ultimate Amy-DR (250×4.6mm 5μm);
[0087] Mobile phase: acetonitrile-trifluoroacetic acid (100:0.1);
[0088] Detection wavelength: 210nm.
[0089] Flow rate: 0.5ml / min
[0090] Column temperature: 30°C
[0091] Preparation of the test solution: Take an appropriate amount of the test product, weigh it accurately, add mobile phase to make a solution containing 0.2 mg per 1 ml, and shake well to obtain.
[0092] Preparation of mitiglinide calcium reference substance solution: Take an appropriate amount of the reference substance, weigh it accurately, add mobile phase to make a solution containing 0.2mg per 1ml, and shake well.
[0093] Preparation of R-isomer reference substance solution: Take an appropriate amount of R-isomer reference substance, weigh it accurately, add mobile phase to make a solution containing 0.2mg per 1ml, and shake well.
[00...
example 3
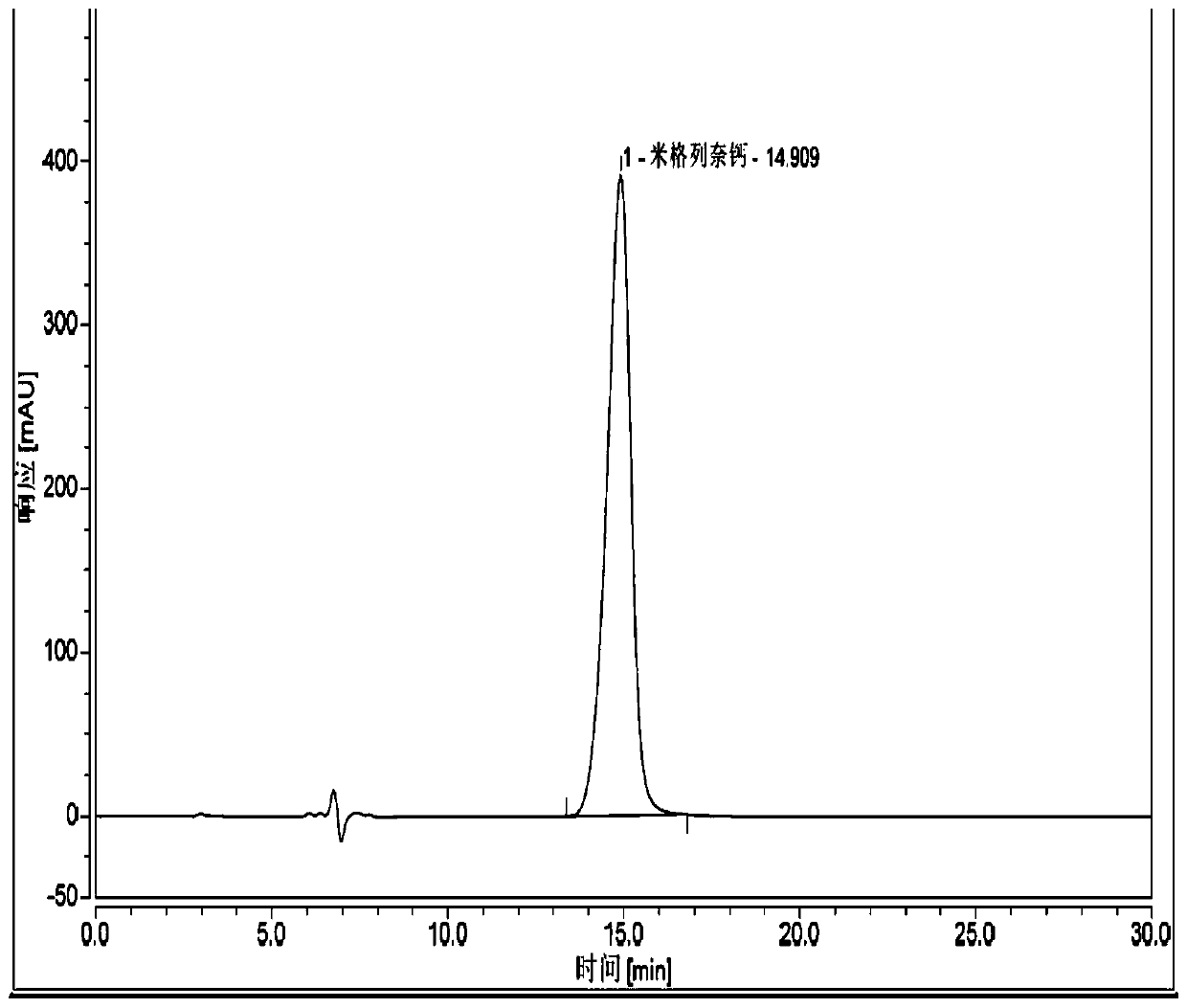
[0097] Chromatographic conditions and system suitability test:
[0098] Chromatographic column: chiral chromatographic column Ultimate Amy-DR (250×4.6mm 5μm);
[0099] Mobile phase: acetonitrile-trifluoroacetic acid (100:0.1);
[0100] Detection wavelength: 210nm.
[0101] Flow rate: 0.8ml / min
[0102] Column temperature: 30°C
[0103] Preparation of the test solution: Take an appropriate amount of the test product, weigh it accurately, add mobile phase to make a solution containing 0.2 mg per 1 ml, and shake well to obtain.
[0104] Preparation of mitiglinide calcium reference substance solution: Take an appropriate amount of the reference substance, weigh it accurately, add mobile phase to make a solution containing 0.2mg per 1ml, and shake well.
[0105] Preparation of R-isomer reference substance solution: Take an appropriate amount of R-isomer reference substance, weigh it accurately, add mobile phase to make a solution containing 0.2mg per 1ml, and shake well.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com